Zinc coordination polymer and preparation method and application thereof
A zinc coordination polymer, a technology of coordination polymers, applied in chemical instruments and methods, instruments, analytical materials, etc., can solve the problems such as the inability of coordination polymer probes to exist stably, achieve high yield and purity, The effect of the production process is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1 zinc coordination polymer
[0024] Weigh the Zn(NO 3 ) 2 ·6H 2 O (59.50mg, 0.20mmol) and DPBA (46.60mg, 0.10mmol) and 4,4'-bipyridine (15.60mg, 0.10mmol) were placed in a 23mL polytetrafluoroethylene reactor liner, and 6.00mL of distilled water was added , adjusted the pH to 5 with 0.50mol / L KOH, stirred at room temperature for 30 minutes, sealed it in a stainless steel reactor, reacted at a constant temperature in a temperature-controllable oven at 120°C for 72 hours, and cooled naturally to room temperature to obtain Colorless massive crystals meeting the requirements of X-ray testing were collected after repeated washing with distilled water and vacuum drying, with a yield of 53%.
Embodiment 2
[0025] The structure determination of embodiment 2 zinc coordination polymer
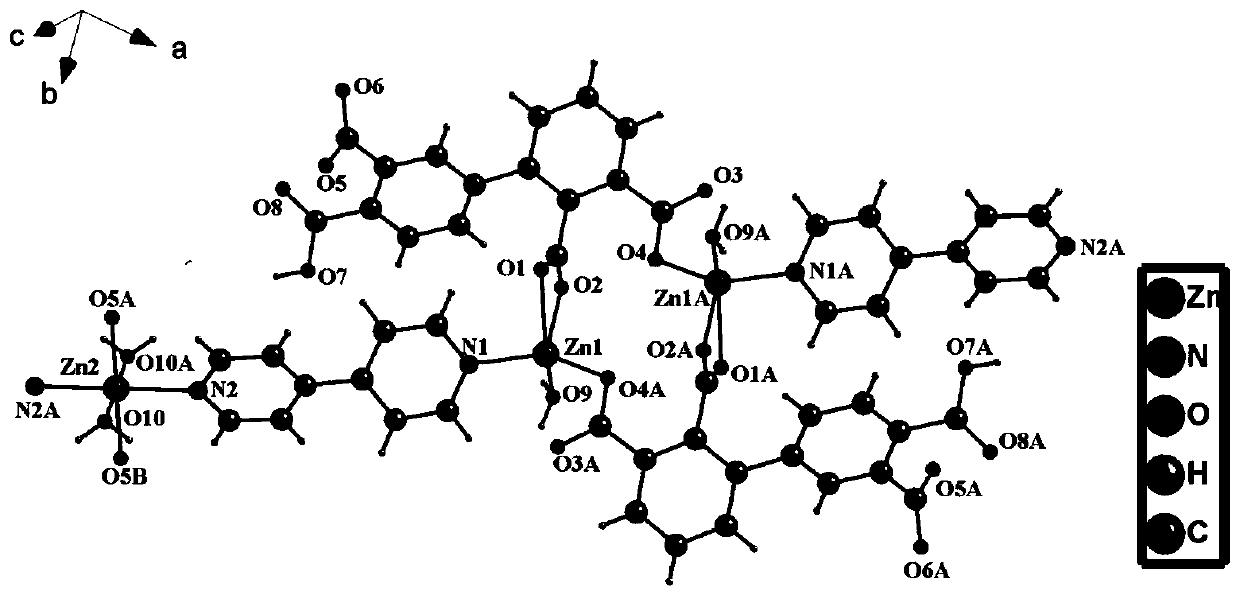
[0026] The crystal structure was determined using the Beijing Synchrotron Radiation 1W2B working line station light source For the incident radiation, the MAR165CCD detector, marccd and HKL2000 programs collected and restored the data, corrected by the least square method to obtain the unit cell parameters, and obtained the crystal structure from the difference Fourier electron density map using the direct method of SHELXL-2014 / 7, and obtained it by Lorentz and polarization effect correction, C and O atoms are hydrogenated using theory and fixed on the parent atom. The detailed crystal determination data are shown in Table 1, and the crystal structure is shown in figure 1 .
[0027] Table 1 Crystallographic data of coordination polymers
[0028]
Embodiment 3
[0029] Embodiment 3 powder diffraction analysis phase
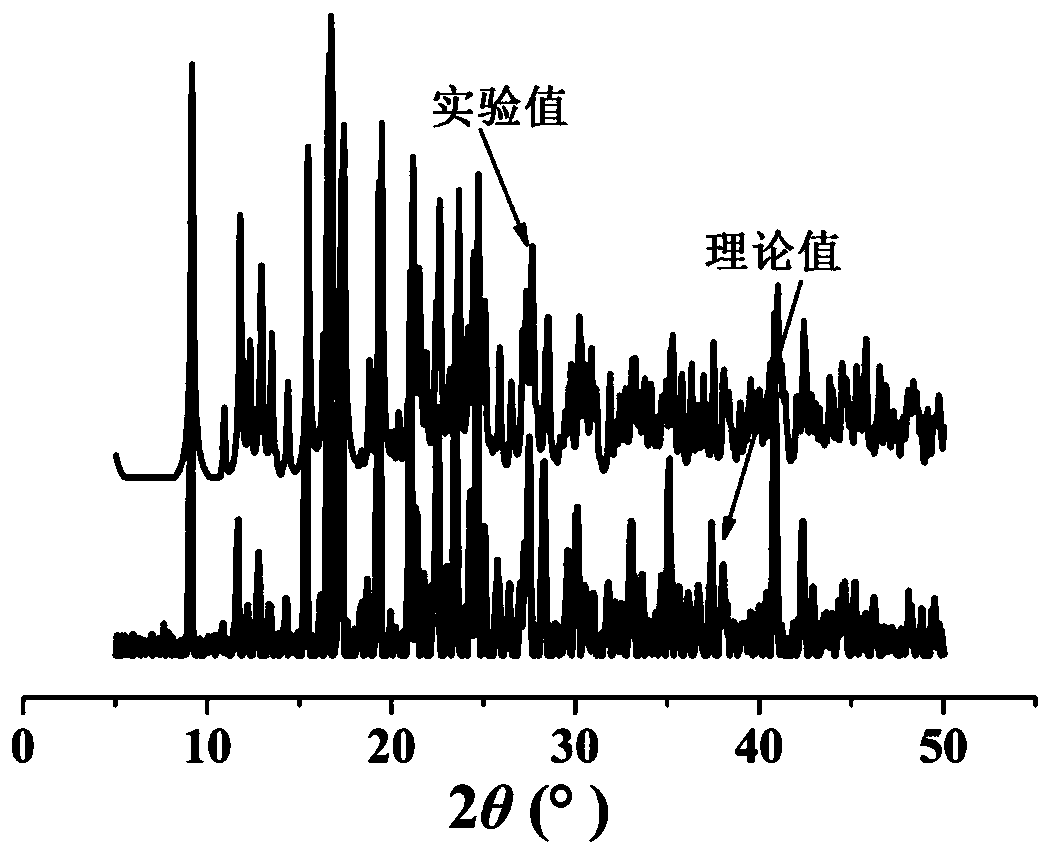
[0030] The X-ray powder diffraction results show that the crystal sample has a uniform phase, and the experimental diffraction pattern is consistent with the powder diffraction pattern simulated based on the crystal structure, see figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com