Combined preparation containing flavone and immunomodulatory peptides, and preparation method and application thereof
An immunomodulatory, flavonoid-containing technology, which is applied in the field of biological nutrition and health care products and pharmaceutical preparations, can solve the problems of ineffective components, short gastric residence time, and poor absorption effect, so as to improve stability and bioavailability degree, improve water solubility and poor stability, and enhance the effect of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] (1) configuring potassium carbonate solution of chrysin (molar concentration is 35mM); (2) configuring molar concentration of 200mM ferric chloride aqueous solution; (3) configuring molar concentration of 10mM immunomodulatory peptide (SEQ ID NO 2: RKDVY ) aqueous solution;
[0077] (4) According to the molar concentration ratio of chrysin:ferric chloride:immunomodulatory peptide is 5:1:1, add it into pure water, the total mass fraction of the final system is 5%; adjust the pH value of the mixed solution to 7.4 , to obtain a blue-brown combined preparation solution. The resulting combined preparation solution was dialyzed and then freeze-dried to obtain a blue-brown powder.
Embodiment 2
[0079] (1) Sodium hydroxide solution (molar concentration is 35mM) of configuring luteolin; (2) Ferric chloride aqueous solution whose molar concentration is 200mM is configured; (3) Immunomodulatory peptide (SEQ ID NO 1 : PGPIPN) aqueous solution;
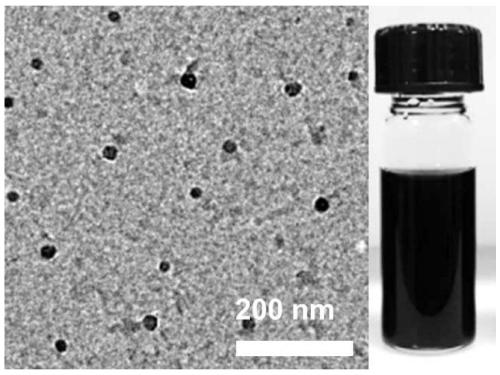
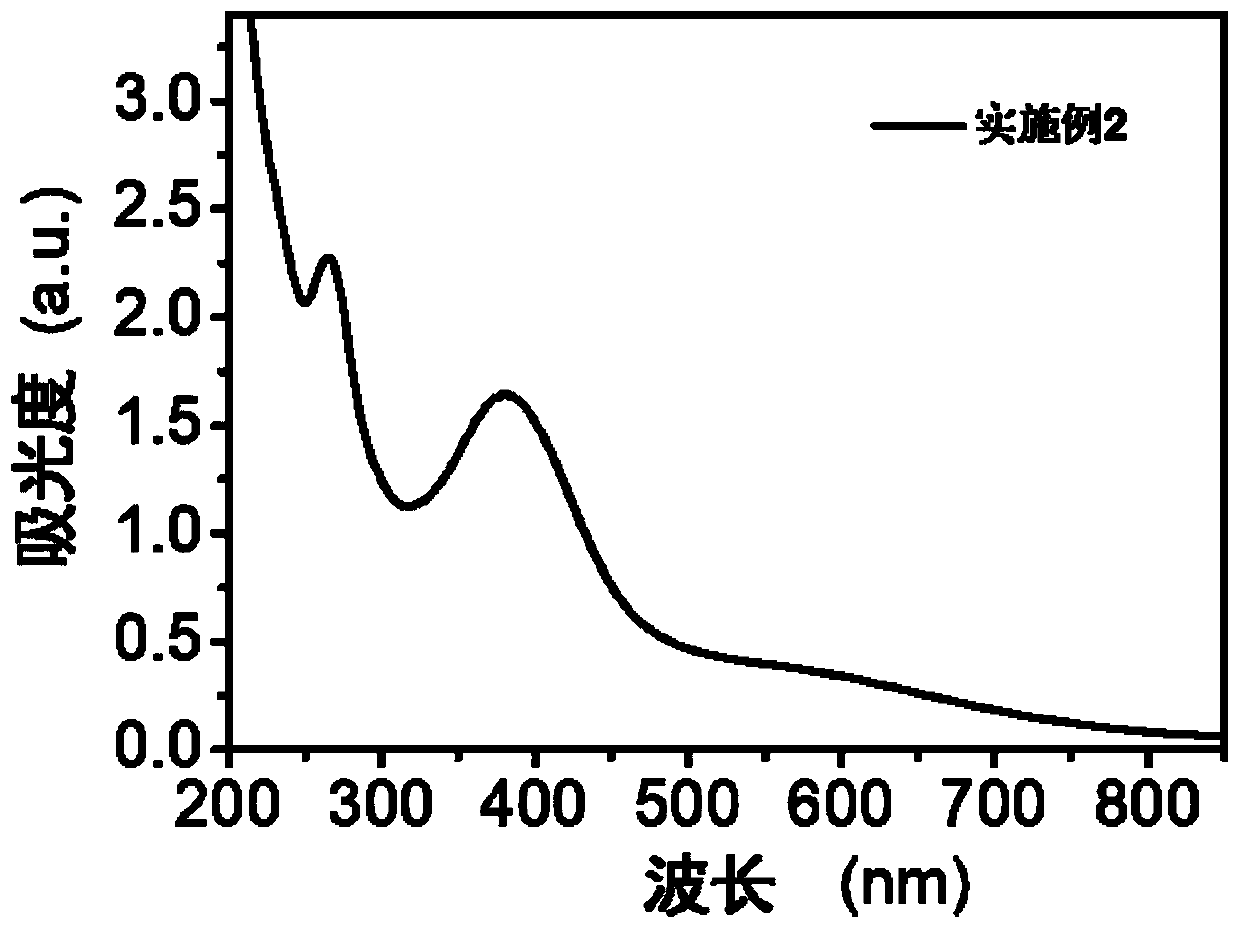
[0080] (4) According to the molar concentration ratio of luteolin:ferric chloride:immunomodulatory peptide is 2:4:1, add it into pure water, the total mass fraction of the final system is 2%; adjust the pH value of the mixed solution to 7.4, to obtain a brown-black combination preparation solution. The transmission electron micrograph and the solution photo of the resulting combined preparation are as follows: figure 1 , the results showed that the prepared combination formulations were uniform in size and had good dispersibility. Measure the absorption spectrum of combination preparation with ultraviolet spectrophotometer, the result is as follows: figure 2
[0081] The resulting combined preparation solution was dialyzed an...
Embodiment 3
[0083](1) configure the sodium hydroxide solution (molar concentration is 70mM) of nobietin; (2) configure the molar concentration of 10mM ferrous chloride aqueous solution; (3) configure the molar concentration of 1mM immunomodulatory peptide (SEQ ID NO 5 : LLY) aqueous solution;
[0084] (4) According to the molar concentration ratio of nobietin: ferrous chloride: immunomodulatory peptide is 5:1:1, add it into pure water, the total mass fraction of the final system is 0.1%; adjust the pH value of the mixed solution to 6.5, to obtain a brown combination preparation solution.
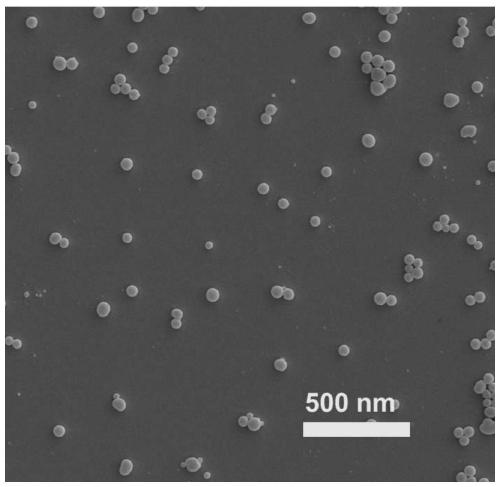
[0085] The scanning electron micrograph of gained combination preparation and solution photo are as follows image 3 , the results showed that the prepared combination preparation was granular and uniform in size, and the particle size was consistent with the DLS results.
[0086] The resulting combined preparation solution was dialyzed and then freeze-dried to obtain a brown powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com