Preparation method of MOF-metal nanoparticle-COF-based composite material
A technology of metal nanoparticles and composite materials, which is applied in the field of preparation based on MOF@metal nanoparticles@COF composite materials, to achieve high dispersion, simple preparation method, stable and firm combination of electrostatic interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
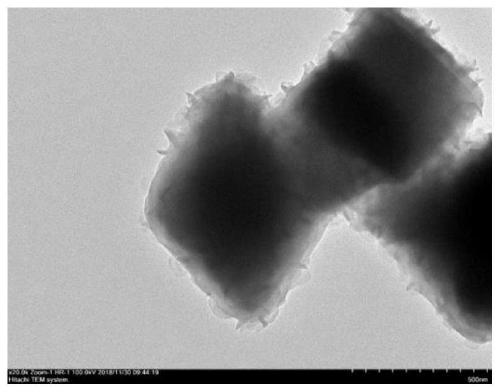
Image
Examples
Embodiment 1
[0028] 1) Dissolve 1mmol of titanium tetraisopropoxide and 3mmol of 2-aminoterephthalic acid into a mixed solution containing 9ml of DMF solution and 1ml of methanol, stir evenly with a magnetic force and place in a 50ml polytetrafluoroethylene high-temperature reaction kettle In 150°C high temperature reaction for 72h, wash and centrifuge with DMF and methanol solution successively, then vacuum dry at 80°C for 12h to obtain MIL-125(Ti)-NH 2 Material;
[0029] 2) 0.4g prepared MIL-125(Ti)-NH 2 The material was dispersed in 10ml of solvent deionized water, and 0.8ml of chloroauric acid solution (Au 3+ content of 5 mg / ml), stirred at room temperature for 4 hours, added 30 mg of sodium borohydride, stirred at room temperature for 4 hours, centrifuged, washed with deionized water three times, and dried in an oven at 80°C to obtain MIL-125(Ti)-NH 2 @AuComposite;
[0030] 3) 0.2g prepared MIL-125(Ti)-NH 2 After the @Au composite material was activated at 80°C for 12 hours, it wa...
Embodiment 2
[0034]1) Mix 4mmol of ferric nitrate, 1mmol of 2-aminoterephthalic acid and 10ml of DMF solution evenly and place it in a 50ml polytetrafluoroethylene high-temperature reaction kettle, react at 150°C for 6 hours, and then wash with DMF and methanol solution in turn Centrifuge, then vacuum dry at 80°C to obtain amino-modified MIL-53(Fe)-NH 2 Material;
[0035] 2) 0.3g prepared MIL-53(Fe)-NH 2 The material was dispersed in a mixed solution of 30ml ethanol and water (the volume ratio of ethanol: water was 4:1), and 1mL potassium chloroplatinate (Pt 2+ The concentration is 3mg / ml) aqueous solution, and then the above solution is stirred under the ultraviolet light for 3 hours at low temperature by the ultraviolet light reduction method, and finally centrifuged, washed with deionized water for 3 times, and dried at 80°C to obtain MIL-53(Fe)-NH 2 @PtComposite;
[0036] 3) 0.1g of prepared MIL-53(Fe)-NH 2 After @Pt material was activated at 100°C for 10 hours, it was dispersed in...
Embodiment 3
[0040] 1) Mix 0.675g of ferric chloride, 0.225g of 2-aminoterephthalic acid and 15ml of DMF solution evenly and place them in a 100ml polytetrafluoroethylene high-temperature reactor. Wash and centrifuge with methanol solution, then vacuum dry at 80°C to obtain amino-modified MIL-101(Fe)-NH 2 Material;
[0041] 2) 0.2g prepared MIL-101(Fe)-NH 2 The material was dispersed in 20ml of n-hexane, and 0.6ml of chloroauric acid solution (Au 3+ Concentration is 5mg / ml), after stirring at room temperature for 2h, let it stand, take out n-hexane, dry at room temperature and then in a tube furnace in 5%H 2 / Ar atmosphere and reduction treatment at 200°C for 2h, after cooling to obtain MIL-101(Fe)-NH 2 @AuComposite;
[0042] 3) 0.2g of prepared MIL-101(Fe)-NH 2 The @Au material was activated at 120°C for 12 hours and dispersed in 15ml of a mixed solution of 1,4-dioxane and mesitylene with a volume ratio of 2:1, and then added 0.06mmol of 2,2'-bipyridine- 5,5'-diformaldehyde was ultr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com