Polypeptide molecule for preventing PD-L1 from redistributing on tumor surface, and preparation and application of polypeptide molecule
A molecular and polypeptide sequence technology, applied in the field of medicine, can solve problems such as redistribution, poor tumor permeability, and large molecular weight, and achieve the effects of avoiding redistribution, reducing antibody systemic toxicity, and improving blocking effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the design of target molecule
[0045] In the following examples, the polypeptide sequence is synthesized by the polypeptide solid-phase synthesis method, and the experimental materials required for the synthesis include: dimethylformamide (DMF), piperidine, Wang resin, dichloromethane (DCM), ninhydrin reaction Reagents (ninhydrin, vitamin C and phenol), benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU), hexahydropyridine, triisopropylsilane ( TIS), ethanedithiol (EDT), anhydrous ether, trifluoroacetic acid (TFA), N-methylmorpholine (NMM), N-fluorenylmethoxycarbonyl-6-aminocaproic acid (Fmoc-e-Acp -OH), methanol, amino acid, peptide solid phase synthesis tube, etc.
[0046] In the following examples, the following solutions need to be prepared when using the polypeptide solid-phase synthesis method: deprotection solution - mix hexahydropyridine and DMF at a volume ratio of 1:4; reaction solution - mix NMM and DMF at a volume ratio of 1...
Embodiment 2
[0054] Example 2: Verification of Aggregation
[0055] In this example, the aggregation property is verified by using the polypeptide molecules provided in Example 1.
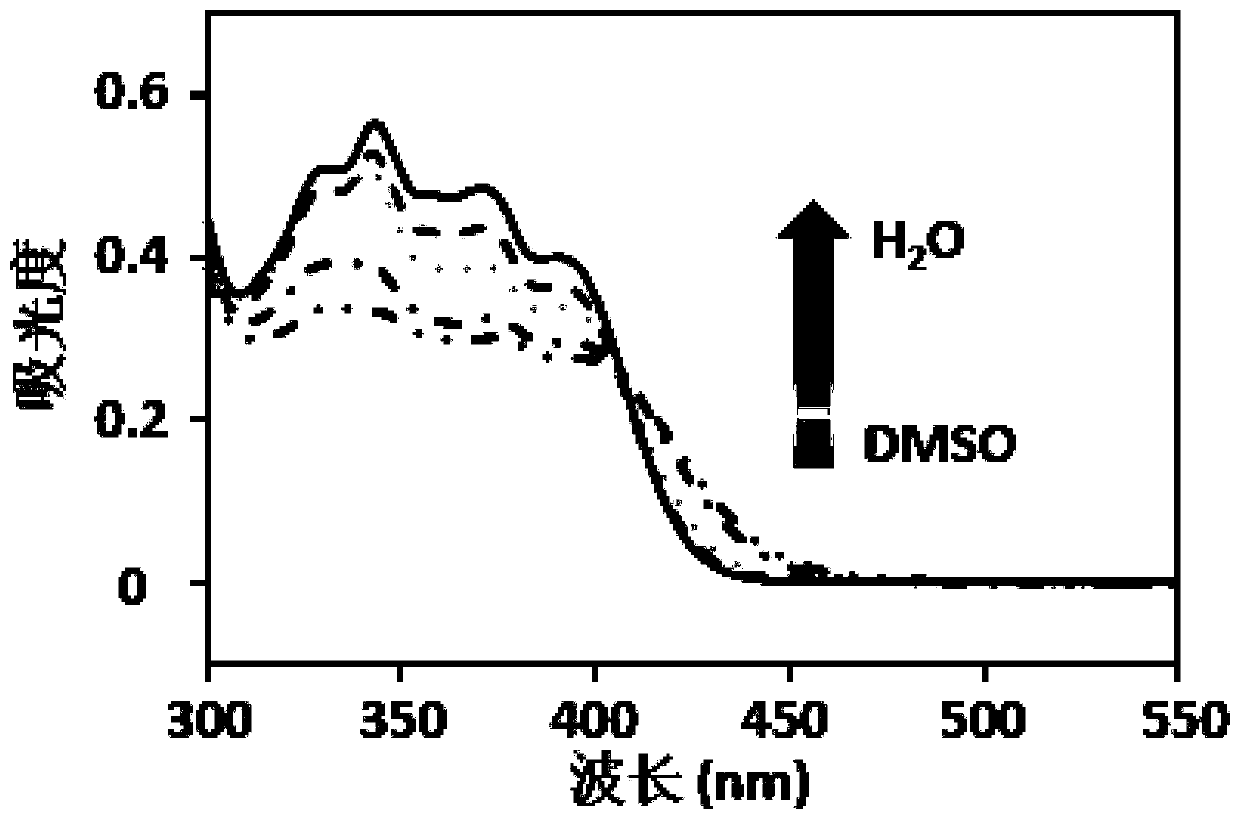
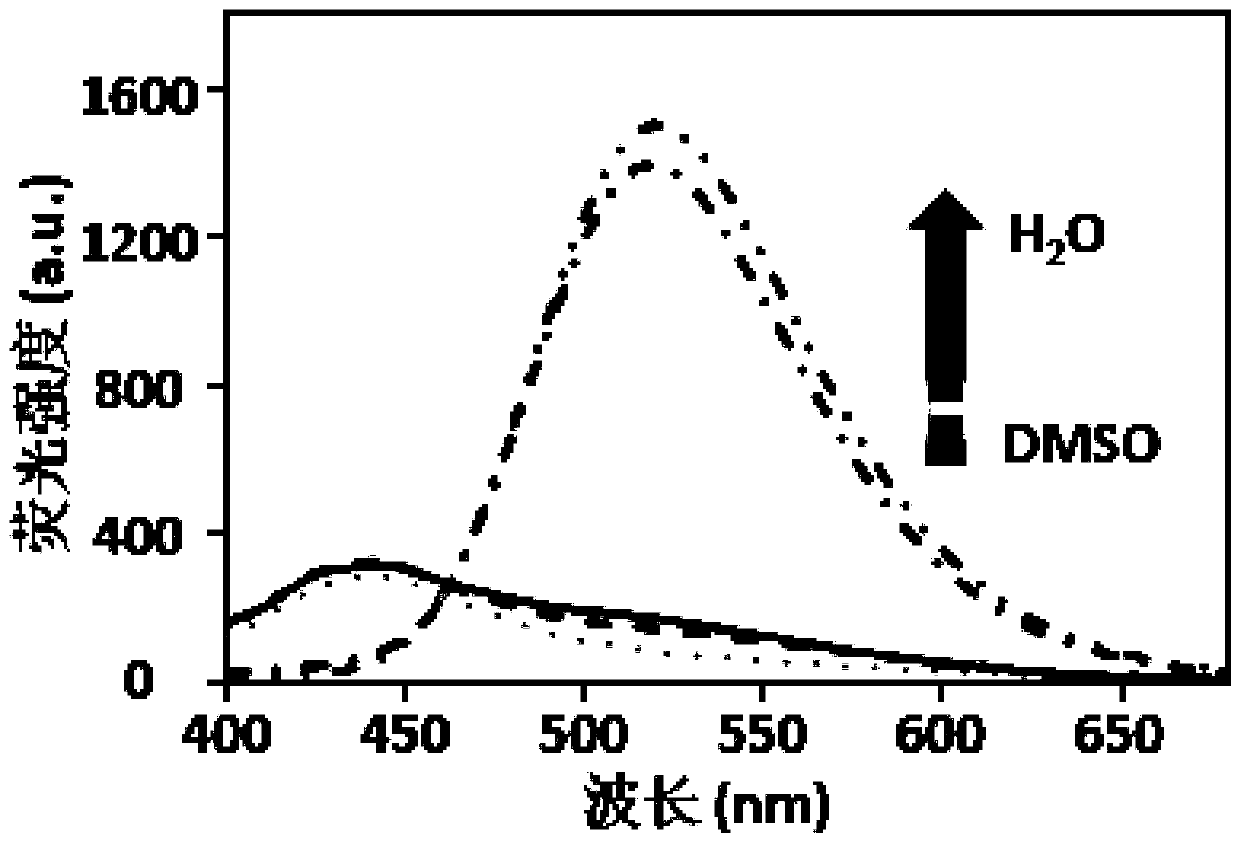
[0056] The experimental steps are as follows: dissolve the polypeptide molecules in dimethyl sulfoxide (DMSO) to prepare the mother solution, make it fully dispersed, and present a monodisperse state. By mixing the volume of water and DMSO, prepare mixed solutions with different ratios of 0:100, 10:90, 30:70, 50:50, 70:30, and 90:10, and the concentration of the material is 3.0×10 -5 M. A UV spectrophotometer (LAMBDA650, U.S., PerkinElmer, USA) was used to detect the absorbance of the target molecule at different water contents, and the absorbance-wavelength curve was drawn. The result is as figure 1 As shown, there is a peak at 350 and an intersection at 410. With the increase of the water content, the material showed a gradually decreasing trend in the range of 410-300 nm, a rising trend in the range of 4...
Embodiment 3
[0057] Example 3: Verification of Assembly Conditions
[0058] In this example, the polypeptide molecules provided in Example 1 are used to verify the assembly properties.
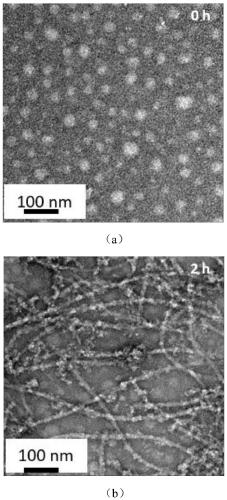
[0059] The morphology of the polypeptide molecules was observed with a transmission electron microscope (lanthanum hexaboride transmission electron microscope, Tecnai G2 20 S-TWIN (T-20), American FEI Company). The selected concentration is 3.0×10 -5 M target molecule solution, when PD-L1 protein is not added, such as image 3 (a) shows the morphology of nanospheres. After the protein was added, the nanospheres disintegrated and gradually self-assembled to form nanofibers. Such as image 3 (b) shows that at 2 h, through the interaction of intermolecular hydrogen bonds, III interaction occurs to form nanofibers.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com