KRAS high-expression cancer vaccine based on recombinant attenuated Listeria monocytogenes and preparation method and application method thereof
A technology for Listeria and cancer vaccines, applied in the field of biomedicine, can solve the problem of low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
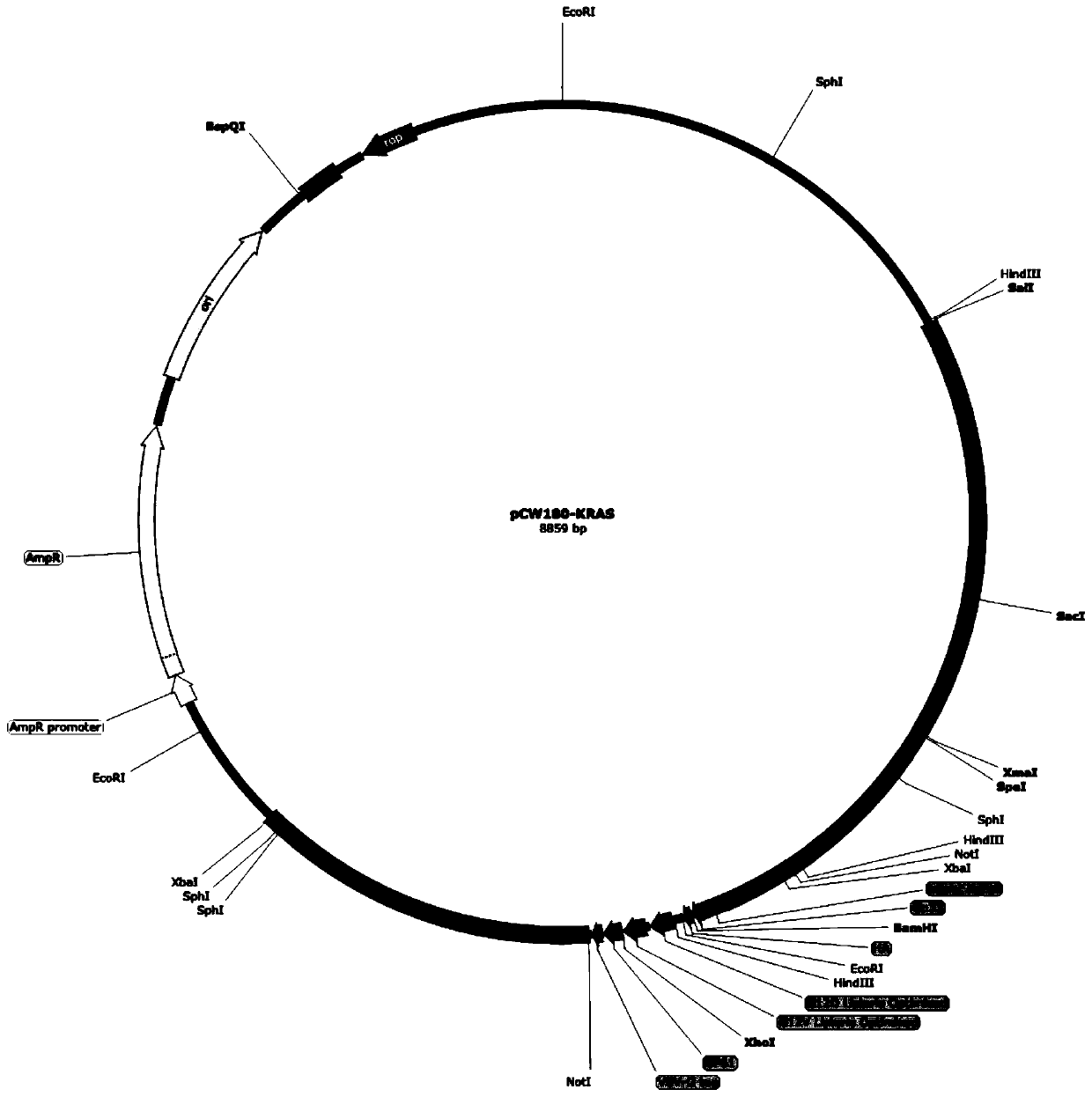
[0059] The preparation method of recombinant plasmid pCW180 is as follows:
[0060] In step a, the Lm mpl gene carrying SpeI upstream and NotI downstream is inserted between the SpeI and NotI restriction sites of the plasmid pCW154 to obtain the first intermediate recombinant plasmid pCW160, the gene sequence of which is the sequence Shown in SEQ ID NO.4 in the list;
[0061] Step b, inserting the Lm orfBAldh gene carrying XbaI upstream and NotI downstream between the XbaI and NotI restriction sites of the first intermediate recombinant plasmid pCW160 to obtain the second intermediate recombinant plasmid pCW170, the second intermediate recombinant plasmid pCW170 The gene sequence is shown in SEQ ID NO.5 in the sequence listing;
[0062] Step c, digest plasmid pCW154 with NotI to obtain a gene with NotI restriction sites at both ends, and insert it into the NotI restriction site of the second intermediate recombinant plasmid pCW170 to obtain recombinant plasmid pCW180.
[006...
Embodiment 1
[0096] Embodiment 1. Preparation of vaccine bacteria LiΔactAplcB-KRAS;
[0097] (1) KRAS gene fragment synthesis;
[0098] Query the KRAS (G12D, G12V mutation) protein gene from NCBI, then optimize it according to the codons of Listeria, add a HindⅢ restriction site upstream, add a XhoⅠ restriction site downstream, and finally obtain the DNA sequence directly by synthesis (obtained in the form of cloning plasmid pUC57-KRAS provided by the company), (the sequence is shown in sequence 1 in the sequence listing).
[0099] (2) Construct targeting plasmid;
[0100] ① Construction of intermediate plasmid pCW203-KRAS
[0101] Plasmids pUC57-KRAS and pCW203 were extracted according to the instructions of the plasmids, and eluted with 30 μL Elution Buffer. pUC57-KRAS and pCW203 were double-digested with HindⅢ and XhoⅠ, restriction enzyme digestion system 20 μL: pUC57-KRAS or pCW2032 O Make up the system to 20 μL. Digest the plasmid in a water bath at 37°C for 1 hour, add 0.5 μL CIA...
Embodiment 2
[0138] Example 2: Preparation of the vaccine strain LmΔactAplcB-KRAS
[0139] (1) KRAS gene fragment synthesis; same as Example 1 (1).
[0140] (2) Construct targeting plasmid;
[0141] ①The construction of the intermediate plasmid pCW203-KRAS is the same as in Example 1(2)①
[0142] ②Construction of targeting plasmid
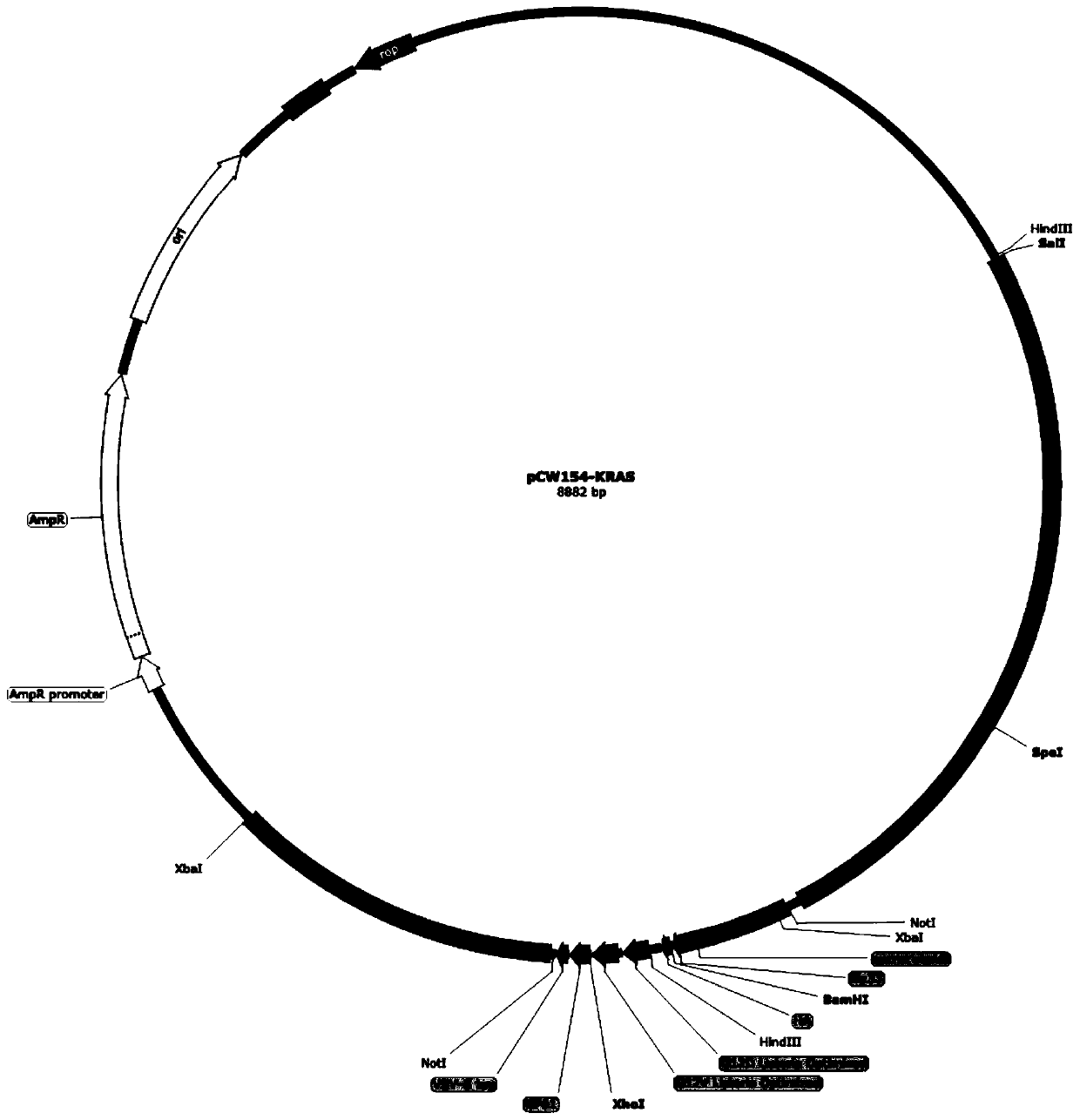
[0143] Plasmid pCW180 was extracted and eluted with 30 μL Elution Buffer. Mix plasmid pCW180 and intermediate plasmid pCW203-KRAS with restriction endonucleases BamHI and XhoI according to the system of (2) ① (replace HindIII enzyme with BamHI enzyme), carry out enzyme digestion and dephosphorylation, and gel recovery after electrophoresis The small fragment after pCW203-KRAS digestion is the insert fragment (244bp) and the pCW180 vector backbone (long fragment after digestion, about 8621bp in length). Mix according to the system of (2)①, connect and transform into Escherichia coli DH5α, smear on LA plate, perform PCR screening, BamHI and XhoI double enzyme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com