Diblock polyester copolymer and process for making
A diblock copolymer and block technology, applied in the direction of one-component copolyester rayon, the structure of seat belts/slings, etc. , low fiber crystallinity and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1-9
[0059] The inventive examples illustrate the effect of catalyst concentration, polymerization temperature profile, and residence time on transesterification. Extruder zone temperatures, extruder screw speed, torque, polyester melt temperature and pressure, vacuum, throughput and residence time are shown in Table I below for all inventive examples. The obtained degree of transesterification is equal to transesterified caprolactone (expressed by σ=4.50ppm) / [caprolactone transesterified (expressed by σ=4.50ppm)+polycaprolactone (expressed by σ=4.24ppm)] . The transesterification in the diblock copolymer as reported in Table II was calculated by multiplying the percent caprolactone in the diblock copolymer by the transesterification.
example 1
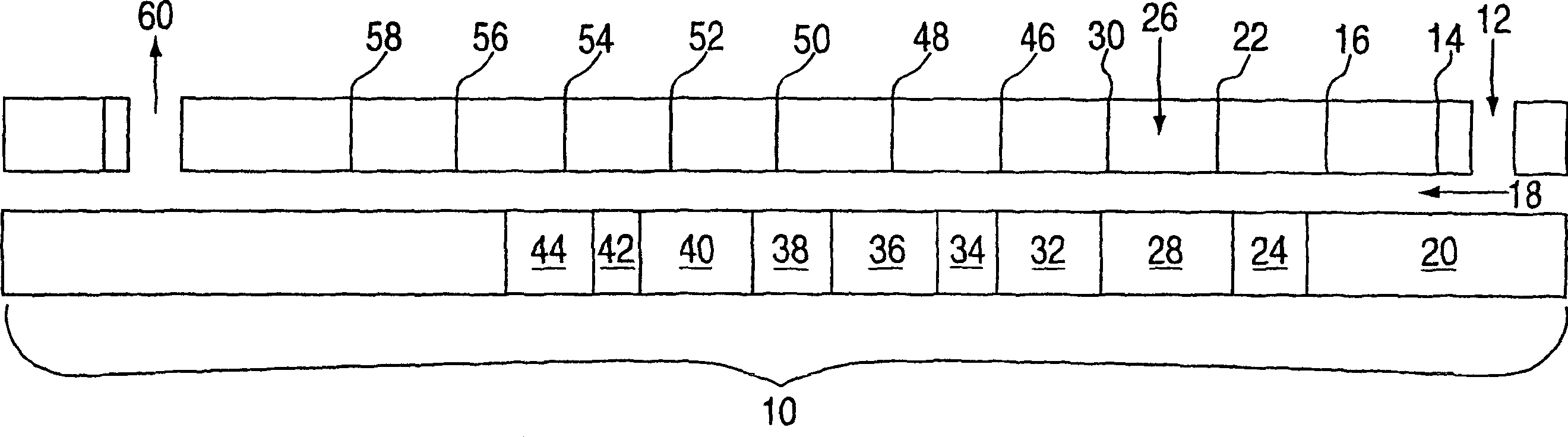
[0061] see figure 1 , dry PET pellets (IV = 0.9, 280°C MV = 15000 poise) were fed into counter-rotating twin-screw extruder 10 (diameter = 27 mm, length = 1404 mm) at feed point 12 at 4.26 lbs / hr. The pellets begin to melt in first zone 14 and second zone 16 and are advanced by pumping element 20 in the direction of arrow 18 to a compression section at third zone 22 . Seal 24 acts as a dynamic seal at the end of the feed zone, tightly compressing the polymer melt and reducing back flow. Each zone is approximately 4 times the screw diameter in length.
[0062] A piston pump injected premixed ε-caprolactone and catalyst (tin octoate, 0.03% by weight of PET-caprolactone) into the extruder at injection point 26 at a rate of 0.75 lb / hr. The amount of ε-caprolactone in PET was 15% by weight. The injected liquid is rapidly mixed back and forth with the PET melt using a distributing and dispersing combing mixer 28 installed in the sprue area. ε-caprolactone solubilizes the PET me...
example 2
[0066] Dry PET pellets (IV = 0.9, 280°C MV = 15000 poise) were fed at feed point 12 at a rate of 7.7 lbs / hr figure 1 A twin-screw extruder 10 is shown. After PET is melted in zones 14 and 16, premixed ε-caprolactone and catalyst (tin octoate, 0.03% by weight of PET-caprolactone) are injected into the melt at injection point 26 at a rate of 2.7 lb / hr . The amount of ε-caprolactone in PET was 26% by weight. With the same extrusion profile as Inventive Example 1 above, the extrusion rate was increased (10.4 lbs / hr) to give an average residence time of 6 minutes. After venting at zone 60, the polymer (PET (74%)-polycaprolactone (26%)) was extruded through a three-hole die, quenched into water, and chopped into pellets. The diblock copolymer has a melting point of 219°C and IV=0.97, which proves that PET and ε-caprolactone have been copolymerized. The transesterification is reported in Table II below.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com