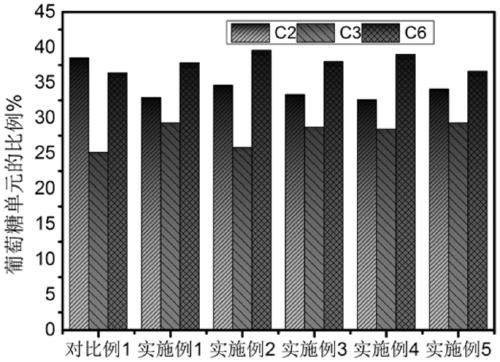
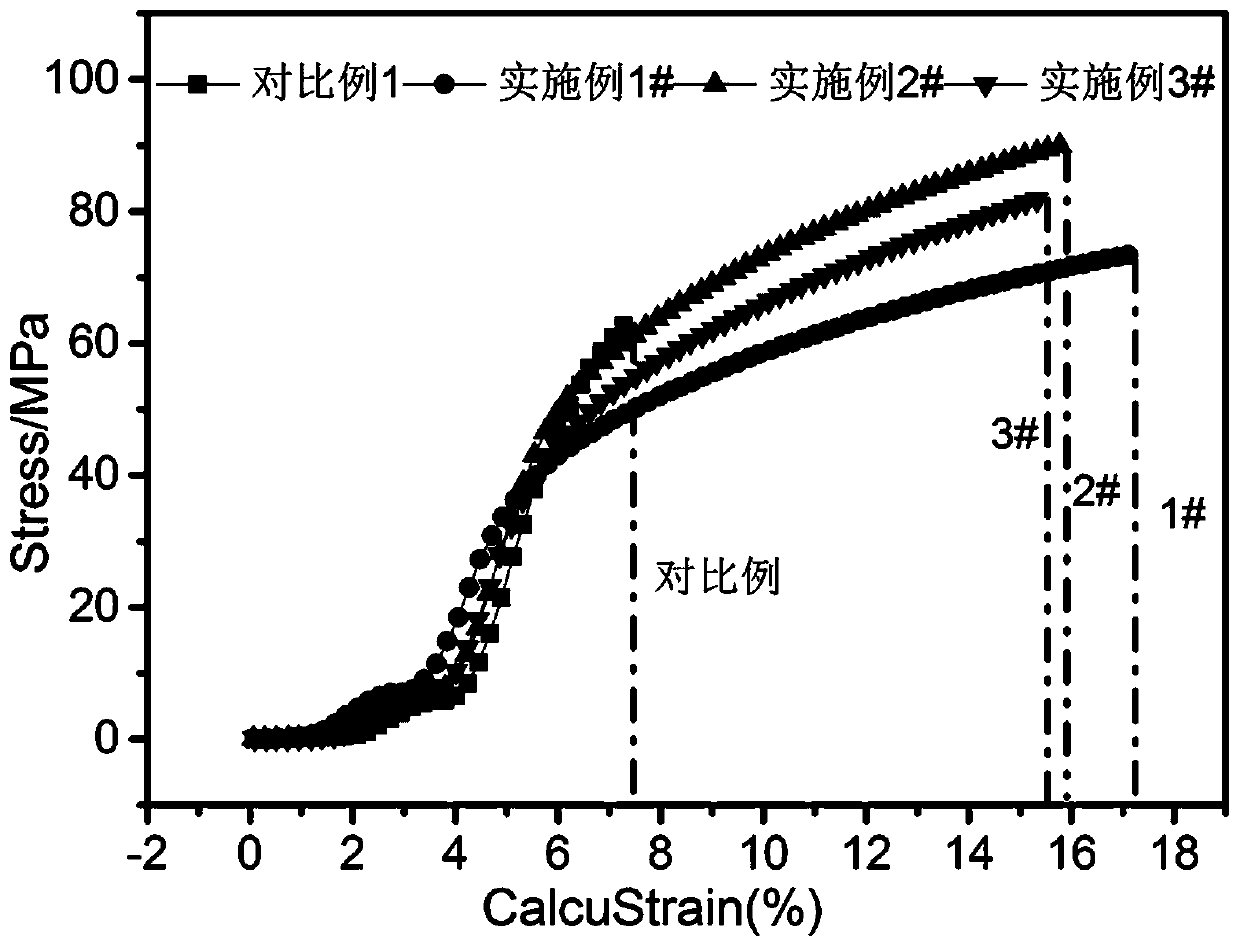
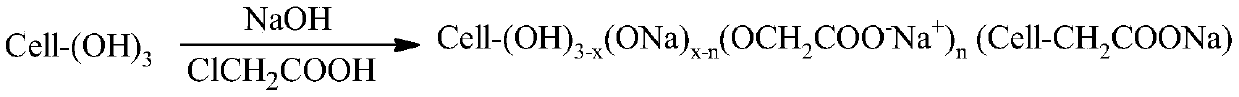Synthesis method of lithium battery binder carboxymethyl cellulose salt
A technology of carboxymethyl cellulose and sodium carboxymethyl cellulose, which is applied in the direction of battery electrodes, circuits, structural parts, etc., can solve the problems of difficult etherification production, insufficient flexibility of alkali fibers, and low elongation at break. Achieve the effects of extremely low residue, complete cellulose reaction and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] In a vertical 5L reactor (vertical reactor), add 220g of cotton, then add 600ml of 10% sodium hydroxide solution and 36g of lithium hydroxide solid, add 2500ml of solvent ethanol, add 89.7ml of chloroacetic acid, React at low temperature for 180 minutes, then raise the temperature to 60°C and react for 180 minutes. After the reaction is over, lower the temperature to 25°C and start centrifuging to discharge the material. After washing, drying, crushing, and screening, the finished product is bagged.
Embodiment 2
[0045] In the vertical 5L reactor (vertical reactor), add cotton 220g, then add 10% sodium hydroxide solution 720ml and lithium hydroxide solid 33.6g, add solvent ethanol 2500ml, add chloroacetic acid 107.6ml, first in 20 React at ℃ for 180min, then raise the temperature to 60℃ and react for 180min, when the reaction is over, lower the temperature to 25℃, start centrifugal discharge, wash, dry, pulverize, sieve, and get the finished product for bagging.
Embodiment 3
[0047] In the vertical 5L reactor (vertical reactor), add cotton 220g, then add 20% sodium hydroxide solution 240ml and lithium hydroxide solid 38.4g, add solvent ethanol 2500ml, add chloroacetic acid 107.6ml, first in 20 React at ℃ for 150 minutes, then raise the temperature to 70℃ and react for 150 minutes. After the reaction is over, lower the temperature to 30℃ and start centrifuging to discharge the material. After washing, drying, crushing and sieving, the finished product is bagged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com