Pt@Ni-SNT/graphene hydrogen evolution catalyst as well as preparation method and application thereof
A catalyst and graphene technology, applied in the direction of metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve problems such as limiting large-scale applications, high cost, and scarce reserves , to achieve the effect of preventing growth and loss, improving dispersion, large economy and social value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
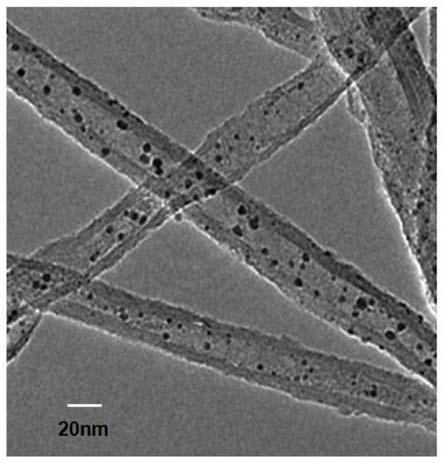
[0025] Preparation of 5wt% Pt@Ni-SNT / Graphene Hydrogen Evolution Catalyst
[0026] (1) 2g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 80ml deionized water, and excess ammonia solution was added dropwise, so that the solution first formed a precipitate, and then the precipitate gradually dissolved. Under magnetic stirring, 0.4mol / l Na was added dropwise. 2 SiO 3 The solution formed a precipitate, wherein the molar ratio of Ni to Si was 0.3; 3wt% polyvinylpyrrolidone was added, and the stirring was continued for 2h, and the resulting mixture was transferred into a polytetrafluoroethylene-lined hydrothermal reactor, and reacted at 180°C for 24h, naturally Cool to room temperature, centrifuge, wash with deionized water and ethanol several times, dry at 150°C, and bake at 350°C to obtain Ni-SNT;
[0027] (2) Disperse the obtained Ni-SNT in an appropriate amount of ethanol, add 0.3M chloroplatinic acid solution, in which the molar ratio of Pt to Ni is 1: 1; ultrasonic 1.5h, so that chl...
Embodiment 2
[0029] 3wt% Pt@Ni-SNT / graphene
[0030] (1) 2g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 80ml deionized water, and excess ammonia solution was added dropwise, so that the solution first formed a precipitate, and then the precipitate gradually dissolved. Under magnetic stirring, 0.2mol / l Na 2 SiO 3 The solution forms a precipitate, wherein the molar ratio of Ni to Si is 0.2; add 3wt% polyvinylpyrrolidone, continue to stir for 0.5h, transfer the resulting mixture into a polytetrafluoroethylene-lined hydrothermal reactor, and react at 220°C for 16h, Naturally cooled to room temperature, centrifuged, washed with deionized water and ethanol several times, dried at 150°C, and calcined at 350°C to obtain Ni-SNT;
[0031](2) Disperse the obtained Ni-SNT in an appropriate amount of ethanol, add 0.3M chloroplatinic acid solution, in which the molar ratio of Pt to Ni is 0.85; ultrasonic 2h, so that chloroplatinic acid is fully filled in the Ni-SNT silicate nano Add 8g / l graphene oxide di...
Embodiment 3
[0033] Electrochemical tests of the catalysts were performed using an electrochemical workstation.
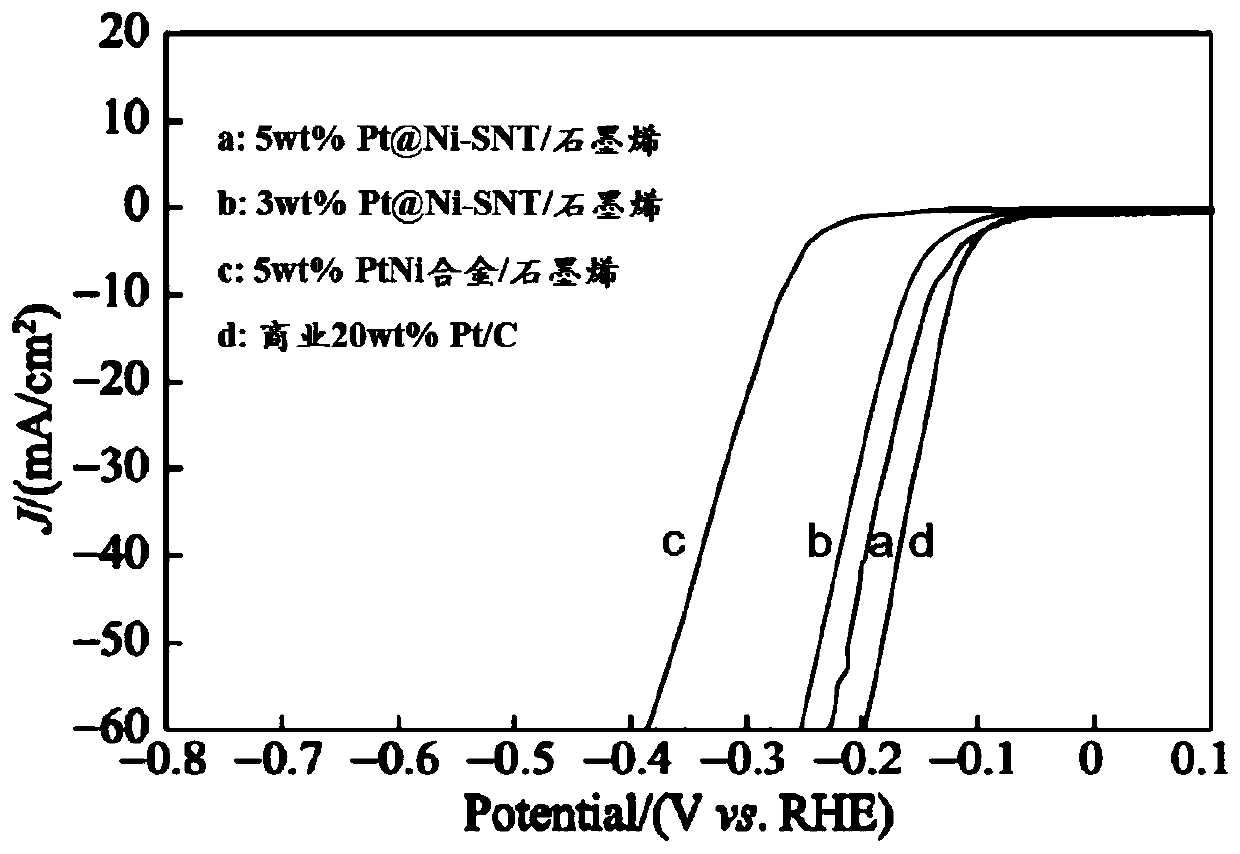
[0034] The electrolyte solution used was 0.5M H 2 SO 4 Solution, the test temperature is room temperature, the test system is a conventional three-electrode system, the Pt wire electrode is used as the counter electrode, and the Ag / AgCl (10wt% KCl) electrode is used as the reference electrode. All potentials in the test results are adjusted relative to the potential of the reversible hydrogen electrode (vs. RHE). The scanning speed of the linear sweep voltammetry (LSV) test is 5mV / s. Before the measurement, the electrodes are scanned 100 times by cyclic voltammetry at a scanning speed of 50mV / s. figure 2 That is the cyclic voltammetry polarization (LSV) curve of the hydrogen evolution catalyst obtained in Examples 1 and 2 of the present invention, as a comparison, also adopt the 5wt% PtNi alloy / graphene hydrogen evolution catalyst recorded in the literature and the commercia...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com