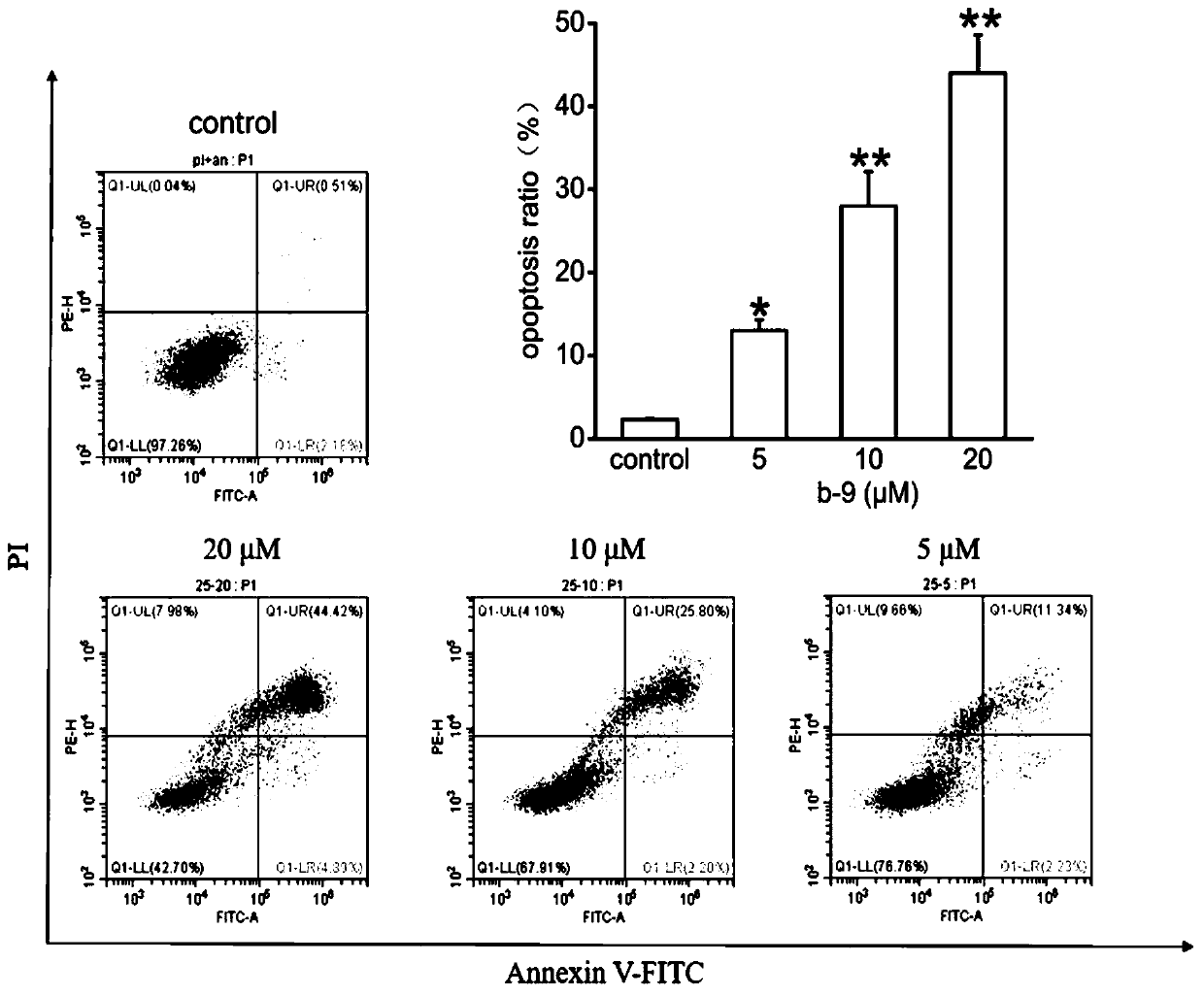
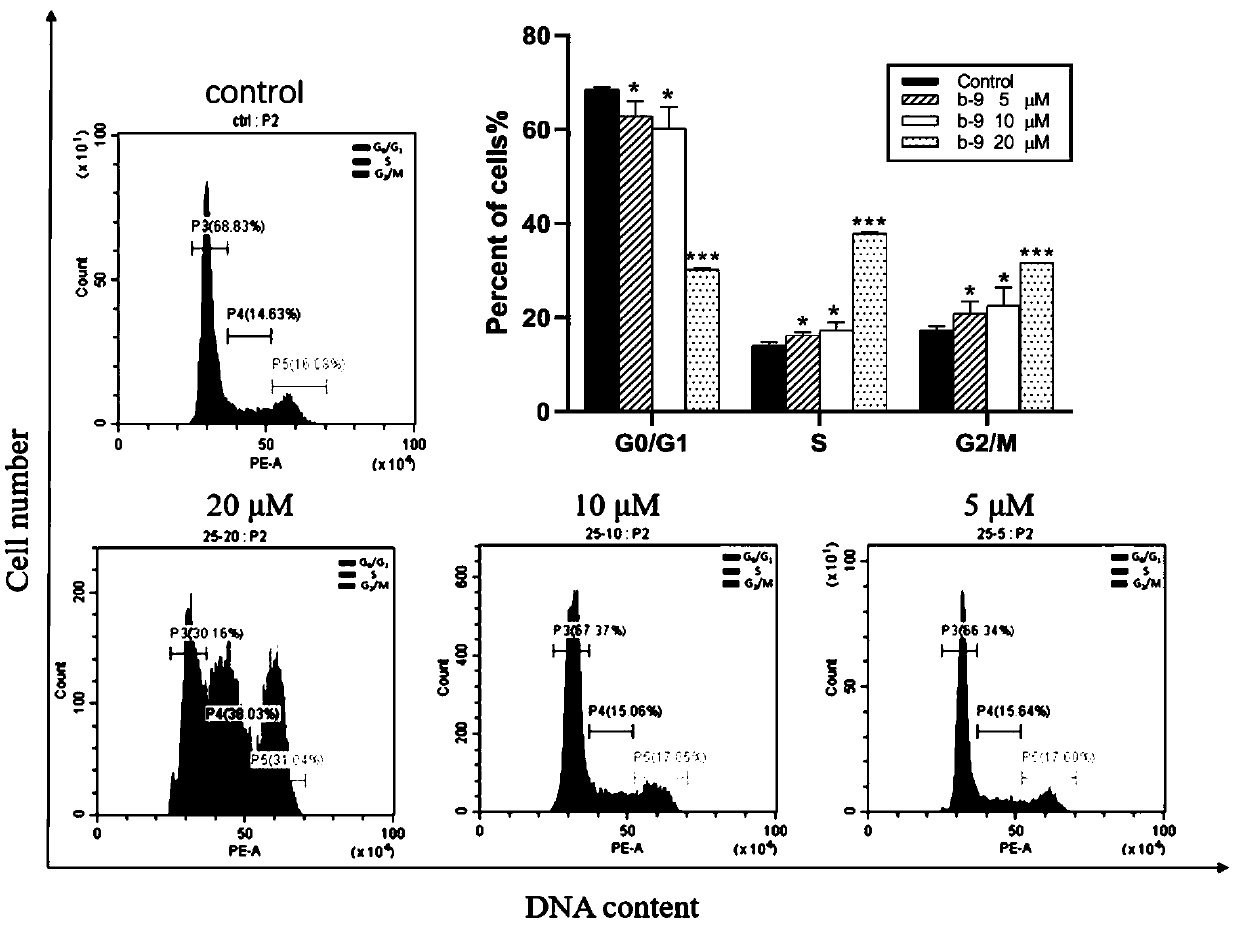
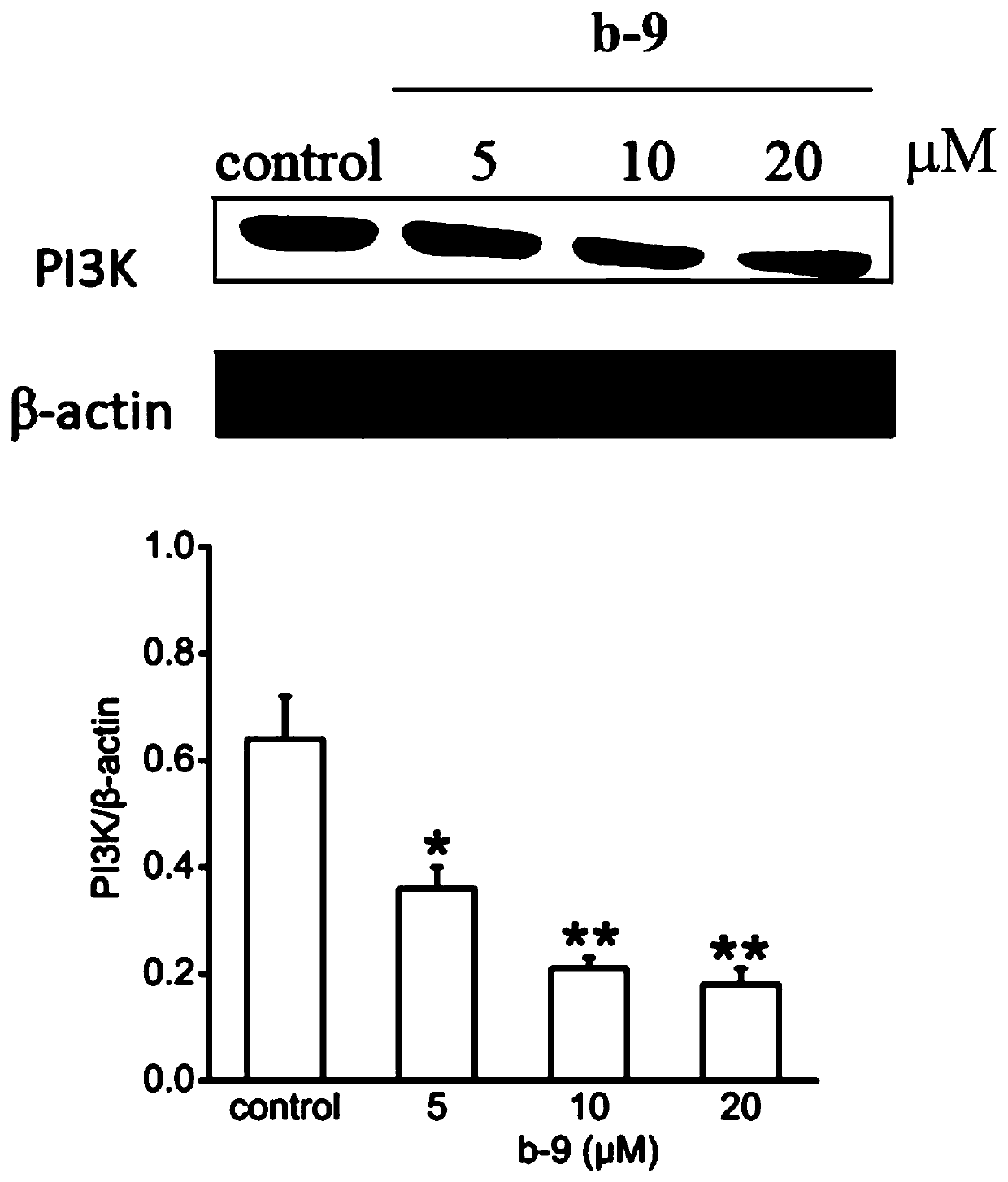
Chrysamide B derivative with anti-tumor activity and preparation and application of Chrysamide B derivative
A technology of anti-tumor activity and derivatives, which can be used in anti-tumor drugs, organic active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation route of carboxylic acid compound (Ⅲ or Ⅵ):
[0055]
[0056] Preparation of Compound II: Dissolve 3g (19.87mmol) benzaldehyde (I) in 40mL ultra-dry dichloromethane in a 250mL reaction flask under argon atmosphere, and slowly add 8.3g (23.84mmol) Wittg reagent (methyl 2-(triphenylphosphoranylidene) propanoate), stirred at room temperature for 12h. After the reaction was detected by TLC, it was diluted with dichloromethane, extracted with water, and the organic phase was collected, dried with anhydrous sodium sulfate, spin-dried, and purified by silica gel column chromatography (V / V PE:EA=15:1 ) to obtain light yellow solid 4g, yield 92%. The same synthetic process is applicable to benzaldehyde, 3-nitrobenzaldehyde, and 2-nitrobenzaldehyde as the starting material (I), and can prepare intermediates (II) with different nitro position substitutions or no substituents; wittig The reagent can also be methyl 2-(triphenyl-l5-phosphaneylidene) acetate, and ...
Embodiment 2
[0062] 2, the preparation route of 5-dimethylpiperazine compound (X):
[0063]
[0064] Preparation of compound 2: (1), in a 500mL reaction flask, 5g (35.97mmol) D-alanine methyl ester hydrochloride (1) and 6.8g (35.97mmol) Boc protected D-alanine were dissolved in In 90mL DMF, move the reaction bottle to an ice bath, and add 20.52g (53.96mmol) 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphoric acid in sequence Salt, 15 mL (107.91 mmol) triethylamine, stirred at room temperature for 16h. It was detected by TLC that the reaction was complete, washed with water, then extracted with ethyl acetate, the organic phase was collected, dried over anhydrous sodium sulfate, and purified by silica gel column chromatography (V / V PE:EA=3:1) to obtain 8.9 g of a white solid. Rate 90%. (2), in a 500mL reaction flask, 5g (35.97mmol) L-alanine methyl ester hydrochloride (VII) and 6.8g (35.97mmol) Boc protected L-alanine were dissolved in 90mL DMF, and The reaction...
Embodiment 3
[0068] Preparation of dimer compounds of formula (two): In a 50mL reaction bottle, mix 100mg (0.45mmol) (2S, 3R)-2-methyl-3-phenyloxirane-2-carboxylic acid (Ⅵ) with 20mg (0.18mmol )(2S,5S)-2,5-dimethylpiperazine (Ⅸ) was dissolved in 2mL of DMF, followed by adding 104mg (0.54mmol) 1-(3-dimethylaminopropyl)-3-ethylcarbodiethylene Amine hydrochloride, 73mg (0.54mmol) 1-hydroxybenzotriazole, 0.19mL (1.08mmol) triethylamine, stirred at room temperature for 16h. The reaction was complete as detected by TLC, washed with water, and the organic phase was collected, dried over anhydrous sodium sulfate, spin-dried, and purified by silica gel column chromatography (V / V PE:EA=1:1) to obtain 75 mg of white solid Chrysamides B, with a yield of 80% . In a 50mL reaction bottle, mix 100mg (0.48mmol) (Z)-2-methyl-3-(4-nitrophenyl) acrylic acid (Ⅲ) with 20mg (0.18mmol) (2S,5S)-2,5-dimethylpiperazine ( Ⅸ) Dissolve in 2mL DMF, then add 104mg (0.54mmol) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com