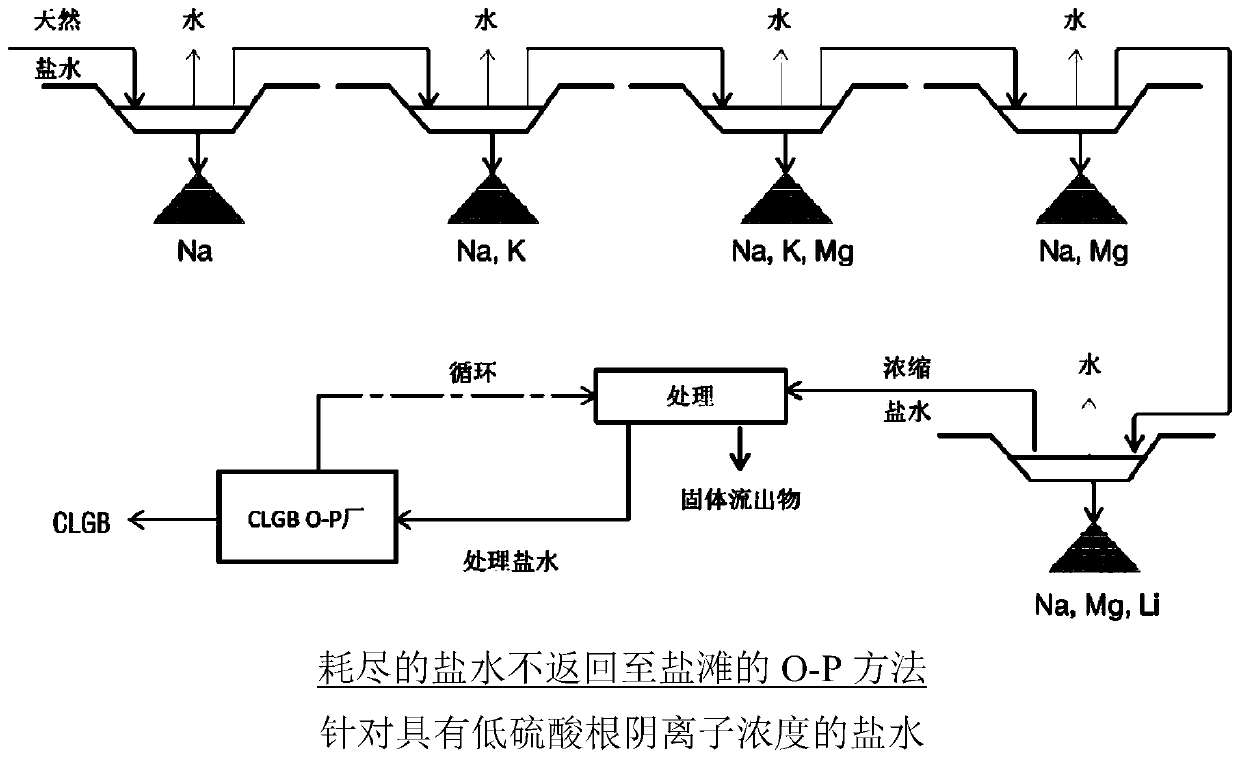
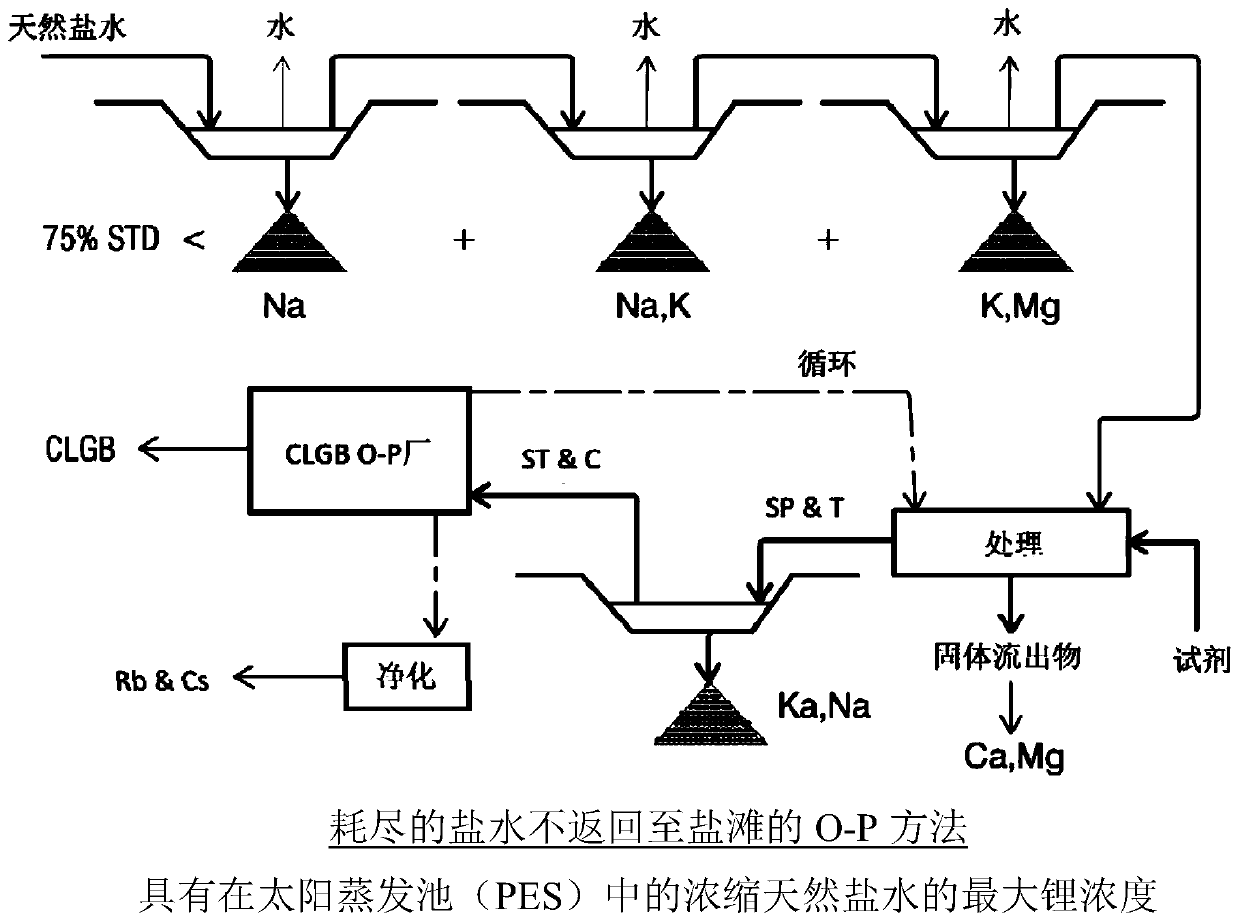
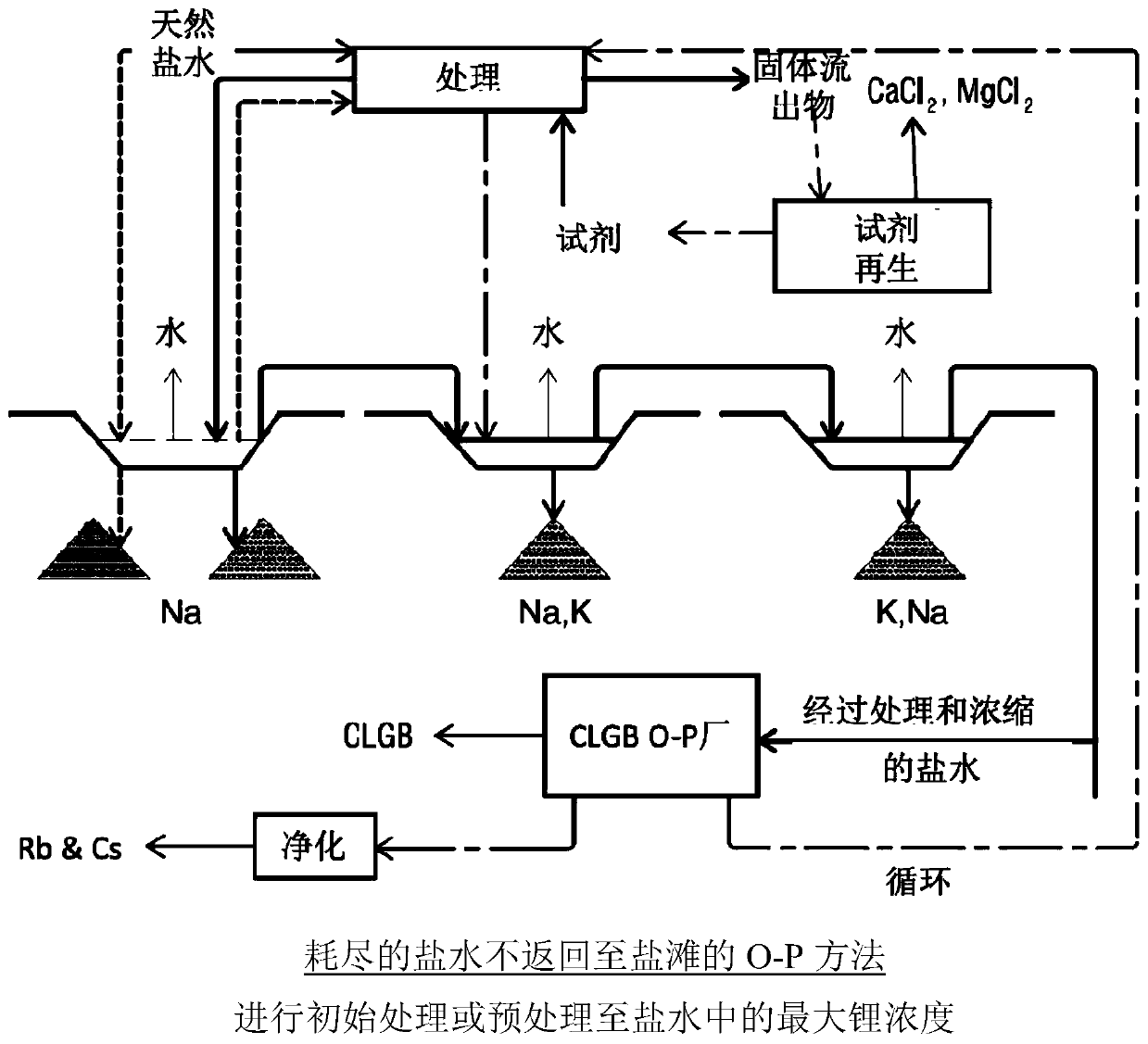
Method for obtaining concentrated brine of minimum impurity content from brine found in natural salt flats and salt marshes, said method having minimum environmental impact and maximum lithium recovery
A technology of impurity content and natural salt, applied in chemical instruments and methods, lithium carbonate;/acid carbonate, lithium compounds, etc., can solve problems such as impossible recovery, many substances of interest, and increased ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0232] Example 1: Natural brine from the salt flats of Diablelos
[0233] The salt flat is a salt mine located in the Puna region of Argentina with an altitude of more than 3,900m.
[0234] Field and laboratory tests
[0235] Phase I: Initial pre-concentration
[0236] On the first day, at an altitude of 4,050m, natural salt water (marked 1 FL(i)) to start the solar evaporation and fractional crystallization process.
[0237] 1 FL(i): the initial liquid phase of stage Ⅰ
[0238]
[0239] 1 V: Volume
[0240] 2 A: Area
[0241] 3 h: height
[0242] 4 STD: total dissolved solids
[0243] 5 ρ: density
[0244] 6 T: temperature
[0245] The chemical composition of the initial liquid phase (FL(i)):
[0246]
[0247]
[0248]
[0249]
[0250] Sub-stage I.1
[0251] The salt water (FL(I)) placed in three ponds was exposed to solar evaporation at an altitude of 4,050m for 10 days. On the 19th day, the precipitated crystals were separated from the liquid phase, and the following results were obtained:...
example 2
[0653] Example 2: Natural brine from Perzueros salt flats
[0654] The salt flat is a salt mine located in the Puna region of Argentina at an altitude of more than 3800 meters.
[0655] Field and laboratory tests
[0656] The lithium content in the natural brine that performs ECL is 0.380g / dm 3 , The limited volume to start the test is 842dm 3 .
[0657] On the first day, 890dm was extracted from the Perzueros salt flat pump 3 Natural brine. The brine (denoted as FL(i)) was transported to the center outside the laboratory in the Tres Moros region, Jujuy Province, Argentina, and the brine was injected into an area of approximately 1.5m the next day 2 The three are similar to the pool. The average height of FL(i) in the pool is approximately equal to 0.197m.
[0658] Natural salt water (FL(i)):
[0659] Weight of FL(i)1,022 kg 1 ρ(FL(i))
1.19kg / dm 3
2 V(FL(i))
890dm 3
3 STD(FL(i))
319g / dm 3
[0660] 1 ρ(FL(i)): liquid phase density of phase I
[0661] 2 V(FL(i)): liquid ph...
example 3
[1047] Example 3: Natural brine from the Rio Grande salt flats
[1048] The salt flat is a salt mine located in the Puna region of Argentina with an altitude of more than 3,800m.
[1049] Field and laboratory tests
[1050] The lithium content in the natural brine that performs ECL is 0.340g / dm 3 , The limited volume to start the test is 835dm 3 .
[1051] On day 1, 840 dm3 of natural brine was extracted by pumping from an existing well in the Rio Grande Salt Marsh. The depth of the well is about 30m. The coordinates that define the location of the well are:
[1052] Latitude: 25°5’27.2”-Longitude: 68°8’23.6”
[1053] The natural brine (denoted as FL(i)) was transported to the center outside the laboratory in the Tres Moros region, Jujuy Province, Argentina, and the natural brine was injected about 1.5m on the second day 2 The three are similar to the pool. The average height in the pool is about 0.186m.
[1054] Natural salt water (FL(i))
[1055] FL(i) quality1,022 kg 1 ρ(FL(i)) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com