Oxygen reduction electrocatalyst material and preparation method thereof
An electrocatalyst and catalytic activity technology, applied in the field of electrocatalytic materials, can solve problems such as deactivation obstacles, high cost nanoparticle catalysts, etc., and achieve the effects of low price, abundant reserves, and diversified synthesis and preparation methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Catalyst preparation
[0037] a. Dissolve 1g of anhydrous zirconium chloride in 2mL of ethanol solution to obtain a clear and transparent solution;
[0038] b. Add 3 g of urea to the solution obtained in step a, and leave it at room temperature for more than 12 hours to obtain a colloidal precursor solution;
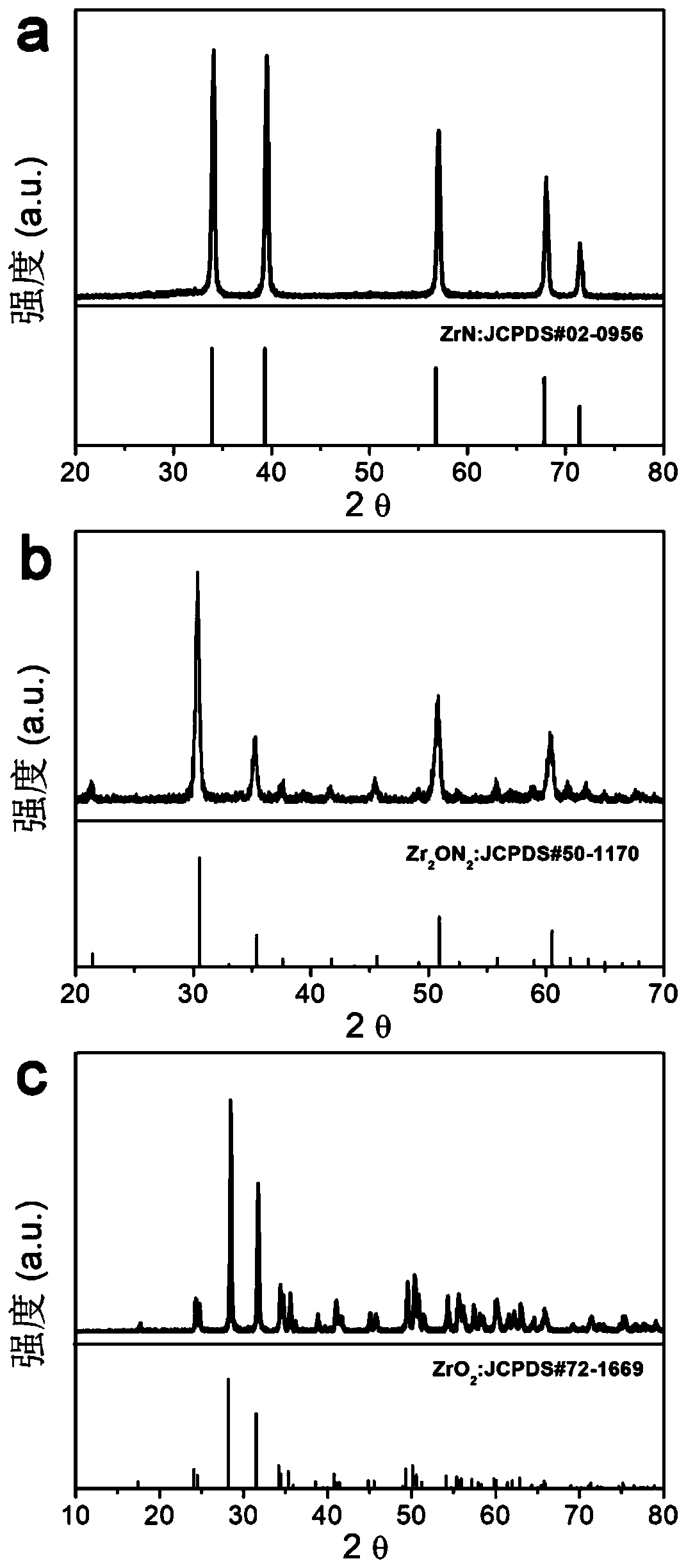
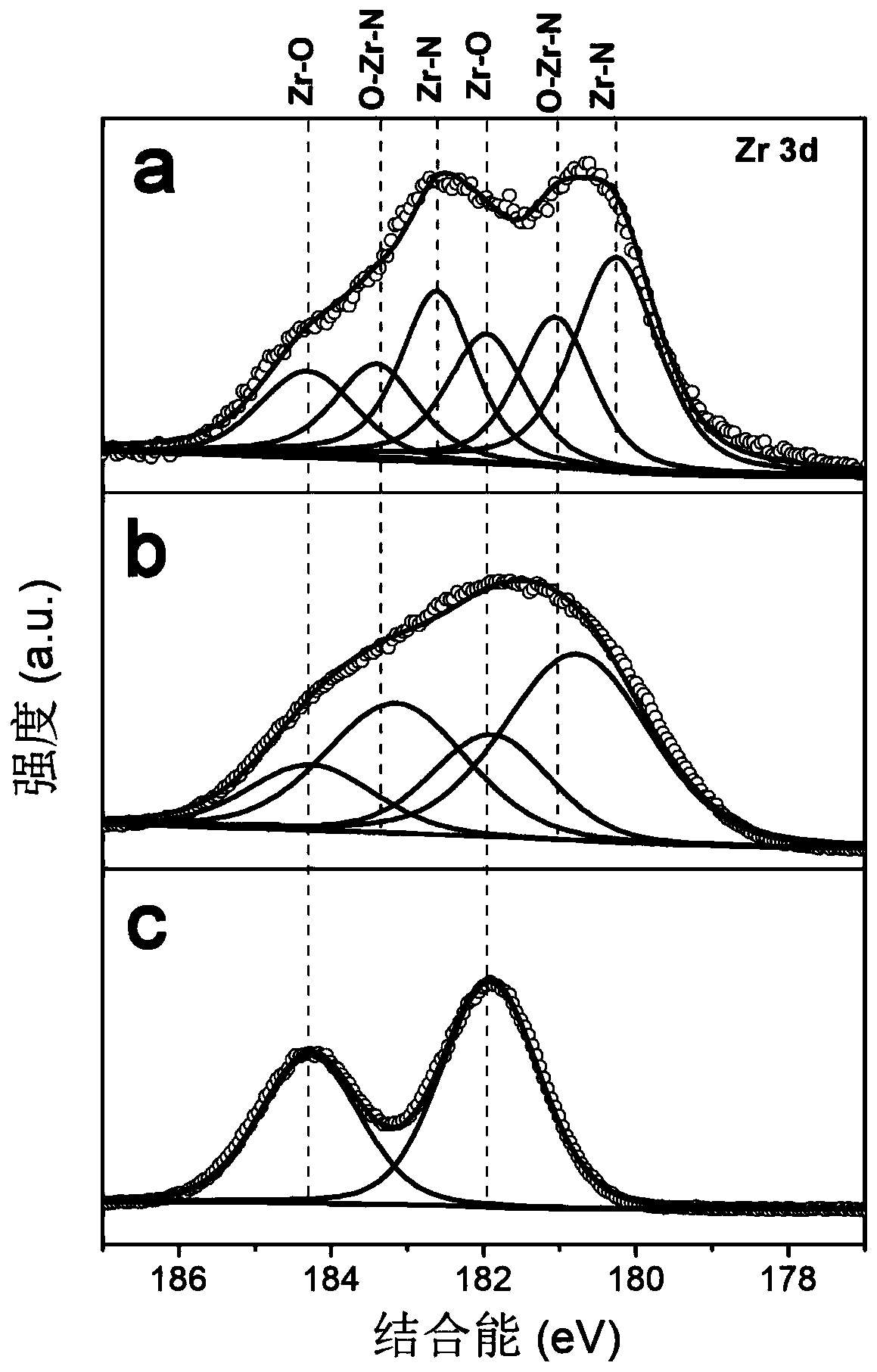
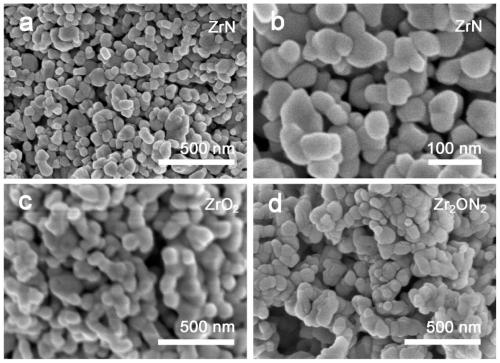
[0039] c. Put the precursor obtained in step b into a sealed tube furnace, and calcinate at 800° C. or higher for 3 hours under an argon / nitrogen atmosphere, with a heating rate of 2° C. / min. Naturally cool down to room temperature to obtain zirconium nitride (ZrN) nanoparticle catalyst. The X-ray diffraction result of the product obtained sees figure 1 , X-ray Photoelectron Spectroscopy see figure 2 , scanning electron microscope image see image 3 ;
[0040] Electrochemical performance test
[0041]Weigh 5 mg of the catalyst powder obtained by the method of the present invention, disperse it in 1 mL of isopropanol aqueous solution containing 0.05% naphtho...
Embodiment 2
[0044] Catalyst preparation
[0045] a. Dissolve 1g of anhydrous zirconium chloride in 2mL of ethanol solution to obtain a clear and transparent solution;
[0046] b. Add 3 g of urea to the solution obtained in step b, and let it stand at room temperature for more than 12 hours to obtain a colloidal precursor solution;
[0047] c. Put the precursor obtained in step b into a sealed tube furnace, and calcinate at 800° C. or higher for 3 hours under an ammonia atmosphere, with a heating rate of 2° C. / min. Naturally cool down to room temperature to obtain zirconium oxynitride nanoparticle catalyst (Zr 2 ON 2 ). The X-ray diffraction result of the product obtained sees figure 1 , X-ray Photoelectron Spectroscopy see figure 2 , scanning electron microscope image see image 3 ;
[0048] Electrochemical performance test
[0049] The test method is the same as in Example 1, except that the surface of the electrode is modified with zirconium oxynitride nanoparticles. For the po...
Embodiment 3
[0051] Catalyst preparation
[0052] a. Dissolve 1g of anhydrous zirconium chloride in 2mL of ethanol solution to obtain a clear and transparent solution;
[0053] b. Add 3 g of urea to the solution obtained in step b, and let it stand at room temperature for more than 12 hours to obtain a colloidal precursor solution;
[0054] c. Put the precursor obtained in step b in a sealed tube furnace, and calcinate at 800° C. or higher for 3 hours in an oxygen / air atmosphere, with a heating rate of 2° C. / min. Naturally lower the temperature to room temperature to obtain a zirconia nanoparticle catalyst (ZrO 2 ). The X-ray diffraction result of the product obtained sees figure 1 , X-ray Photoelectron Spectroscopy see figure 2 , scanning electron microscope image see image 3 ;
[0055] Electrochemical performance test
[0056] The test method is the same as that in Example 1, except that the electrode is modified with a zirconia nanoparticle catalyst. For details on the polariza...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Limiting current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com