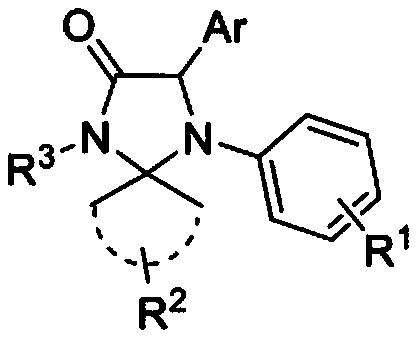
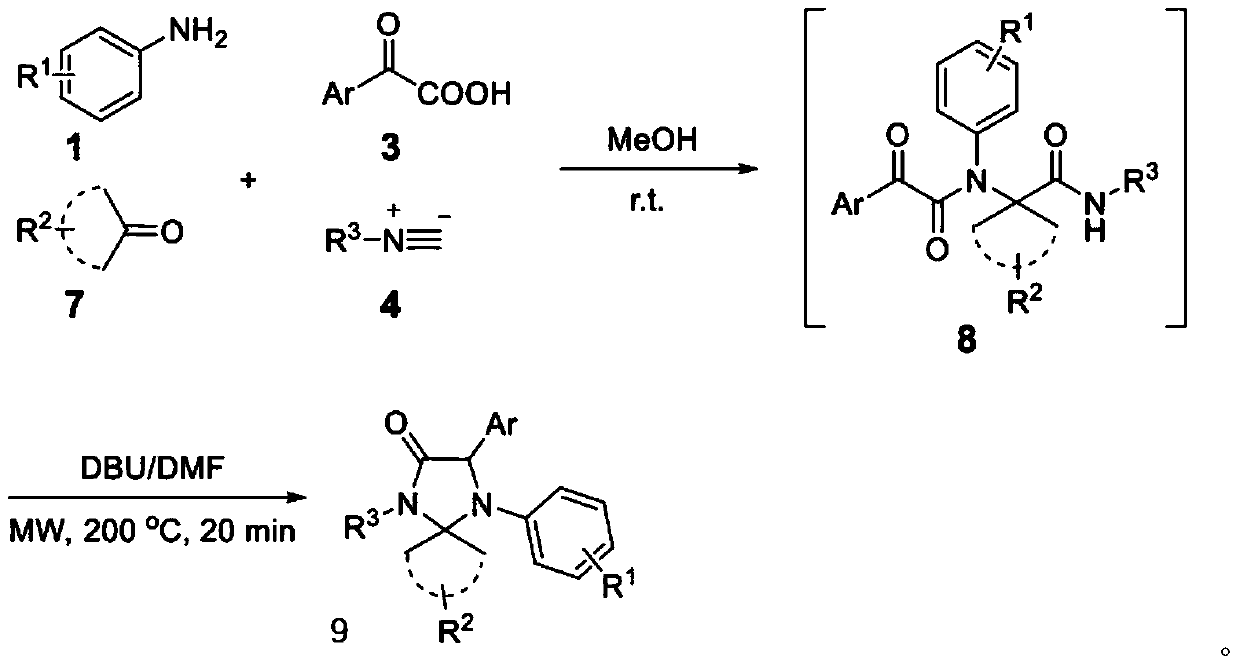
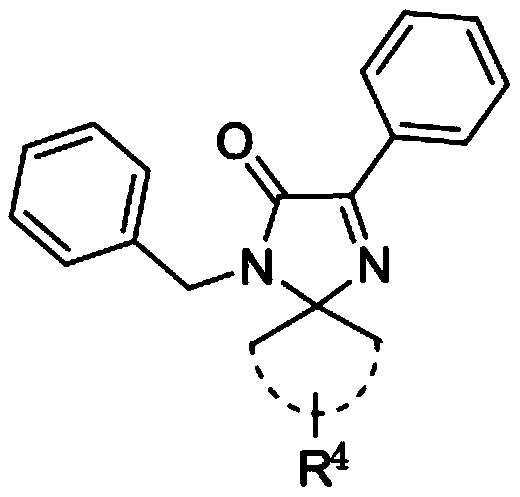
4-imidazolinone derivative and synthesis method and application of method
A technology of imidazolinone and synthesis method, which is applied in the direction of drug combination, organic chemistry, antineoplastic drugs, etc., can solve the problem of carboxylic acid preactivation, etc., and achieve the effect of short synthesis route, simple operation process and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The chemical structural formula of compound 8a is as follows:
[0071]
[0072] The preparation method of compound 8a is as follows:
[0073] Add 0.50mmol cyclohexanone and 0.50mmol 4-bromoaniline to 1mL methanol, and stir in a 5mL microwave bottle for 10 minutes at room temperature, then add 0.50mmol benzoylformic acid and 0.50mmol benzyl isonitrile successively, and mix the obtained The mixture was stirred overnight at room temperature. After the reaction was complete, the disappearance of the isonitrile was detected by TLC. The reaction mixture was concentrated in vacuo to give the crude product, which was then purified by silica gel chromatography (20% EA / Hex) to give 173 mg of compound 8a as a white solid in 67% calculated yield.
[0074] 1 H NMR (400MHz, CDCl 3 )δ7.78–7.69(m,2H),7.52(t,J=7.4Hz,1H),7.43–7.35(m,6H),7.31–7.26(m,5H),6.76(t,J=5.1Hz ,1H),4.62(d,J=5.6Hz,2H),2.27–2.20(m,2H),1.93–1.86(m,2H),1.53–1.52(m,4H),1.40–1.24(m,2H ). 13 C NMR (101MHz, CDCl...
Embodiment 2
[0076] The chemical structural formula of compound 9a is as follows:
[0077]
[0078] The synthesis of compound 9a in this example is carried out according to the synthetic route of 4-imidazolinone derivatives, wherein, R in compound amine 1 1 is bromine, R in compound ketone 7 2 Is cyclohexyl, Ar in compound oxo acid 3 is phenyl, R in compound isocyanide 4 3 is benzyl. The specific synthesis method, raw material formula and process conditions were carried out according to the synthesis method of the related compound 9, and finally the yellow solid compound 9a was obtained with a yield of 60%.
[0079] 1 H NMR (400MHz, CDCl 3 )δ7.60(d,J=7.6Hz,2H),7.38–7.28(m,8H),7.26–7.25(m,2H),7.14(d,J=8.7Hz,2H),5.08(s,1H ),4.68(s,2H),1.94–1.88(m,2H),1.79–1.70(m,2H),1.41–1.22(m,6H). 13 C NMR (100MHz, CDCl 3 )δ171.9, 145.2, 138.2, 137.8, 132.0, 129.3, 128.6, 128.4, 127.7, 127.3, 127.1, 127.0, 118.4, 81.3, 68.4, 44.5, 36.8, 32.5, 24.1, 22.7, 22.4. calcd for C 27 h 28 BrN 2 o + (...
Embodiment 3
[0081] The chemical structural formula of compound 9b is as follows:
[0082]
[0083] The synthesis of compound 9b in this example is carried out according to the synthetic route of 4-imidazolinone derivatives, wherein, R in compound amine 1 1 is methoxy, R in compound ketone 7 2 Is cyclohexyl, Ar in compound oxo acid 3 is phenyl, R in compound isocyanide 4 3 is benzyl. The specific synthesis method, raw material formula and process conditions were carried out according to the synthesis method of related compound 9, and finally brown oily compound 9b was obtained with a yield of 51%.
[0084] 1 H NMR (400MHz, CDCl 3 )δ7.68(d,J=7.5Hz,2H),7.34–7.30(m,6H),7.27–7.22(m,4H),6.77(d,J=8.8Hz,2H),5.06(s,1H ),4.71–4.54(m,2H),3.74(s,3H),1.82–1.54(m,8H),1.18–1.00(m,2H). 13C NMR (100MHz, CDCl 3 )δ172.9,157.7,139.9,138.6,130.8,128.6,128.2,127.4,127.2,127.0,113.9,81.5,69.9,55.3,44.3,37.6,32.6,24.5,22.5,22.1.HRMS(ESI)m / z calcd C 28 h 31 N 2 o 2 + (M+H) + 427.23800, found 427....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com