Indirect ELISA antibody detection kit for African swine fever virus p54 recombinant protein, and preparation method thereof
An African swine fever virus and antibody detection technology, which is applied in the field of African swine fever virus antibody detection and virus detection, can solve problems such as the decline in the accuracy of detection reagents, achieve the effects of simple purification, simple production, and avoid the incidence of cross-reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Preparation of recombinant soluble protein of African swine fever virus p54
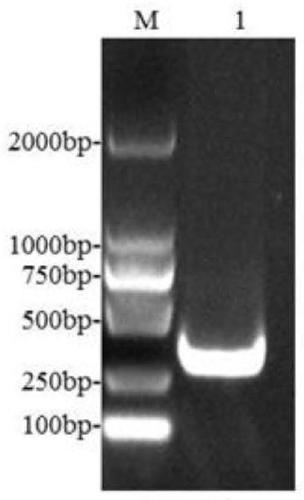
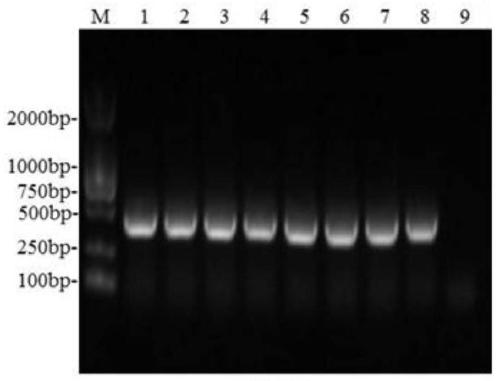
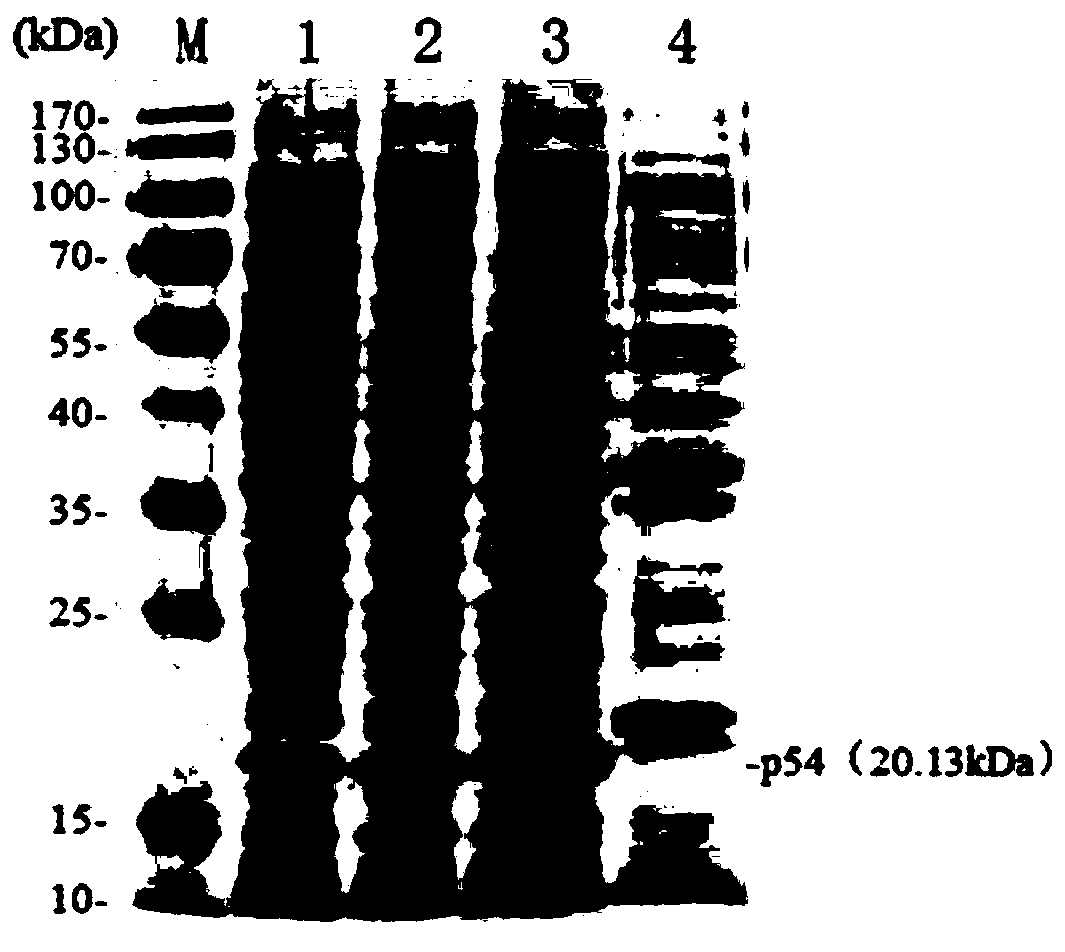
[0043] 1) Amplification of the E183L gene: According to the E183L gene sequence of the ASFV Pig / HLJ / 2018 strain (GeneBank accession number: MK333180.1) determined by the Harbin Veterinary Research Institute of the Chinese Academy of Agricultural Sciences, the specificity of the full-length amplification of the E183L gene was designed using Oligo7 software Primers, the upstream primer used is F:TTAAGAAGGAGATATACATATGTCTTCAAGAAAG (SEQ.NO1); the downstream primer is R:TCAGTGGTGGTGGTGGTGGTGCTCGAGGGATCCACGCGGAACCAGCAAGGAGTTTTTCTAG (SEQ.NO 2). Extract ASFV genomic DNA, utilize PCR method to amplify E183L gene fragment (see figure 1). The PCR amplification system was as follows: 5×HF buffer, 10 μL; upstream primer (10 μmol / L), 2.5 μL; downstream primer (10 μmol / L), 2.5 μL; 100% DMSO, 1.5 μL; 2.5 mmol / L dNTP, 4 μL; Phusion high-fidelity DNA polymerase (2000U / mL), 1μl; recombinant plasm...
Embodiment 2
[0048] Embodiment 2: the determination of optimal process parameter
[0049] 1. Determination of the optimal working concentration of antigen and antibody
[0050] Dilute the purified p54 recombinant protein with coating solution to a concentration of 0.625 μg / ml, 1.25 μg / ml, 2.5 μg / ml, 5 μg / ml, and 10 μg / ml, add it to the microtiter plate from left to right, 100 μl / well, Incubate overnight at 4°C; the next day, wash 3 times with PBST, 5min each time; block with 200μl / well of 5% skimmed milk at 37°C for 2h. The standard negative and positive serum was serially diluted 1:50, 1:100, 1:200, 1:400, 100 μl / well, added to the microtiter plate from top to bottom, reacted at 37°C for 30 minutes, washed 3 times, 5 minutes each time; Add 1:30,000 enzyme-labeled secondary antibody, incubate at 37°C for 45 minutes, wash 3 times, each time for 5 minutes, and pat dry; add 100 μl TMB chromogenic solution to each well, and protect from light at 37°C for 15 minutes; add 100 μl stop solution t...
Embodiment 3
[0063] Embodiment 3 detects the indirect ELISA kit of African swine fever virus p54 antibody
[0064] Prepare the indirect ELISA kit of detecting African swine fever virus p54 antibody of the present invention through the following steps, and its steps are as follows:
[0065] (1) Coating: Coat the microtiter plate with recombinant ASFV p54 protein, the optimal coating concentration of p54 is 2.5 μg / ml, 100 μl / well, incubate overnight at 4°C, wash 3 times with PBST buffer the next day, each 5 minutes each time, pat dry;
[0066] (2) Blocking: add 5% skimmed milk powder, 100 μl / well, block at 37°C for 1.5h, wash 3 times, 5min each time, pat dry; add ELISA plate stabilizer, 100μl / well, block at 37°C for 1h, wash 3 times, 5 minutes each time. (3) Serum incubation: add 1:200 diluted serum to be tested, incubate at 37°C for 30 minutes, wash 3 times, 5 minutes each time, and pat dry;
[0067] (4) Secondary antibody incubation: add 1:30000 enzyme-labeled secondary antibody, incuba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com