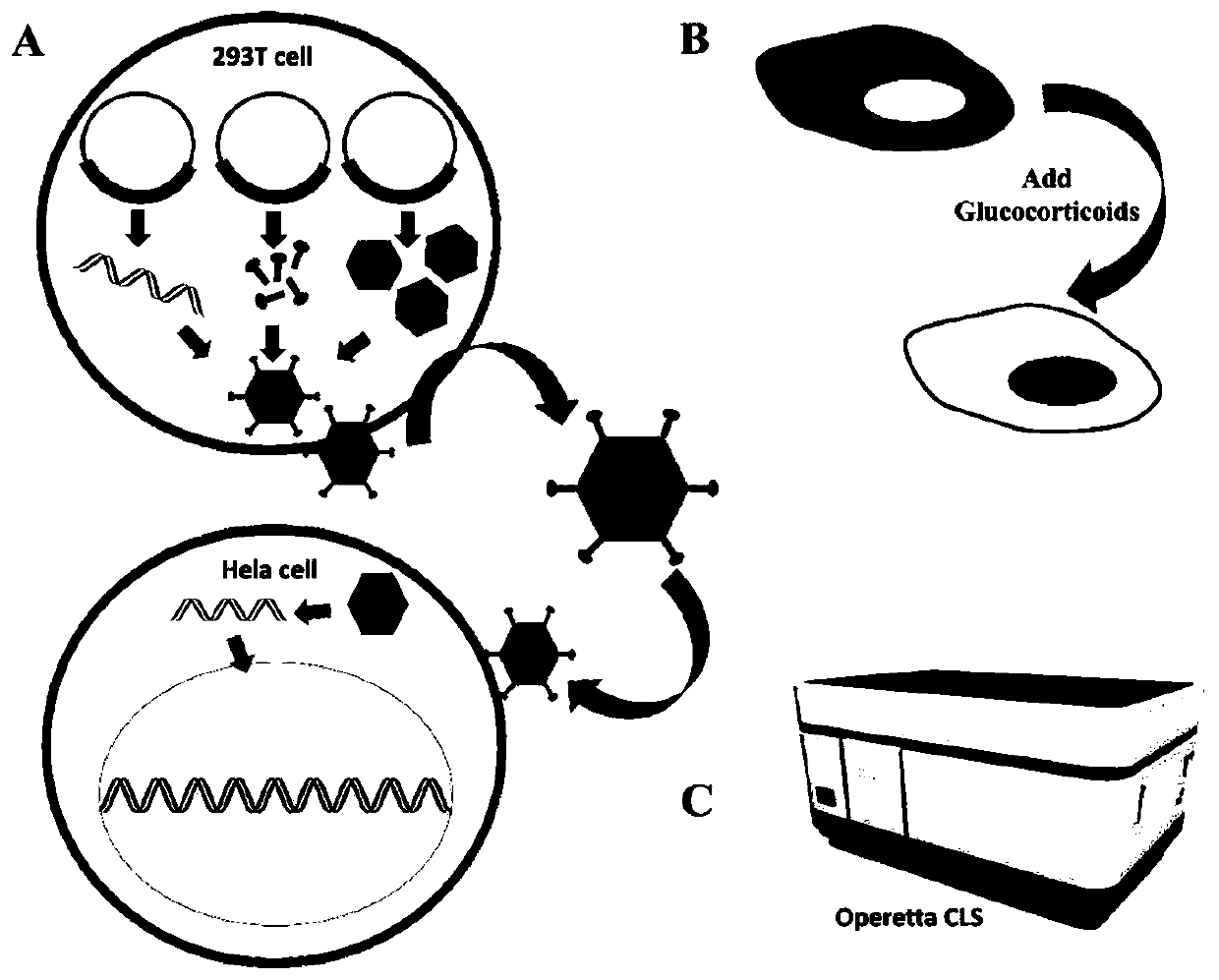
Method for determining glucocorticoid mixture based on transgenic engineering cell strain
A transgenic engineering, glucocorticoid technology, applied in genetic engineering, botanical equipment and methods, biochemical equipment and methods, etc. problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Method for determination of glucocorticoid mixture based on GFP-GR-puro Hela cell transgenic engineering cell line
[0039] 1. Target gene synthesis
[0040] The whole GFP-GR gene (as shown in SEQ ID NO: 1) was synthesized (pTet-GFP-GR) by Nanjing GenScript Company.
[0041] 2. Plasmid extraction and sequencing
[0042] pTet-GFP-GR was transformed into Escherichia coli Trans5α competent cells by heat shock method, spread on LB (Amp) plates, and cultured at 37°C. Pick a monoclonal strain, culture it overnight in 100mL LB (Amp) liquid medium, and use the Gene star endotoxin-free plasmid extraction kit for plasmid extraction.
[0043] Plasmid concentration 857.6ng / μL, OD 260 / 280=1.89; 20uL sample was taken and sent to Sangon for sequencing and identification.
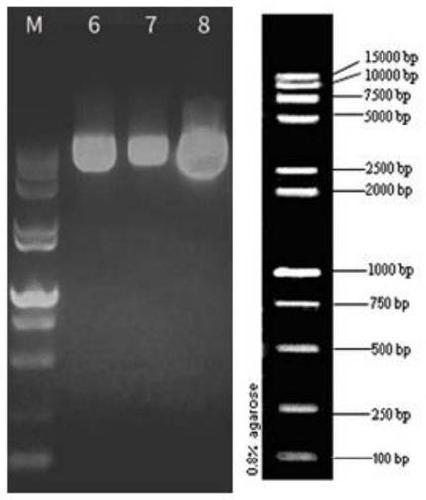
[0044] 3. Carrier Construction
[0045] Sequence the correct plasmid, use PCR to amplify the GFP-GR target fragment, and purify the PCR product; the lentiviral vector plasmid FV026 has been digested and linear...
Embodiment 2
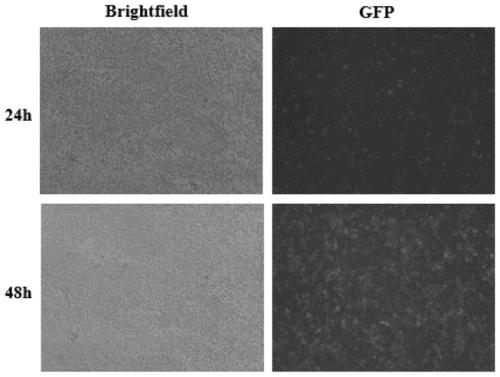
[0060] Testing and validation of glucocorticoid mixtures
[0061] 1. Experimental method
[0062] Perform 17β-estradiol (17β-Estradiol) (negative), dexamethasone (Dexa) (positive) to the stable strain cell GFP-GR-puro Hela that embodiment 1 screens out, and several other glucocorticoid drugs In the administration experiment, the action time was 0.5h, and then the cytoplasmic nuclear migration response was detected.
[0063] In order to eliminate the influence factors in the medium and serum as much as possible, the cell medium was replaced with phenol red-free MEM (+10% activated charcoal-treated serum, 10% CD-FBS) complete medium 1 day in advance. Subsequently, the GFP-GR-puro Hela digestion was counted according to 10 4 Each well was inserted into a black 96-well plate (3603, corning). After culturing overnight, add blank control (DMSO), negative control estradiol (17β-E2), positive drug dexamethasone (Dexa) and several other glucocorticoid drugs (prednisone and methylpre...
Embodiment 3
[0069] 1. Experimental method
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com