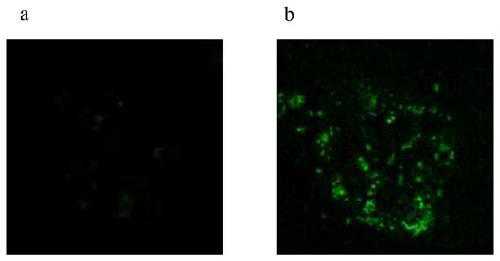
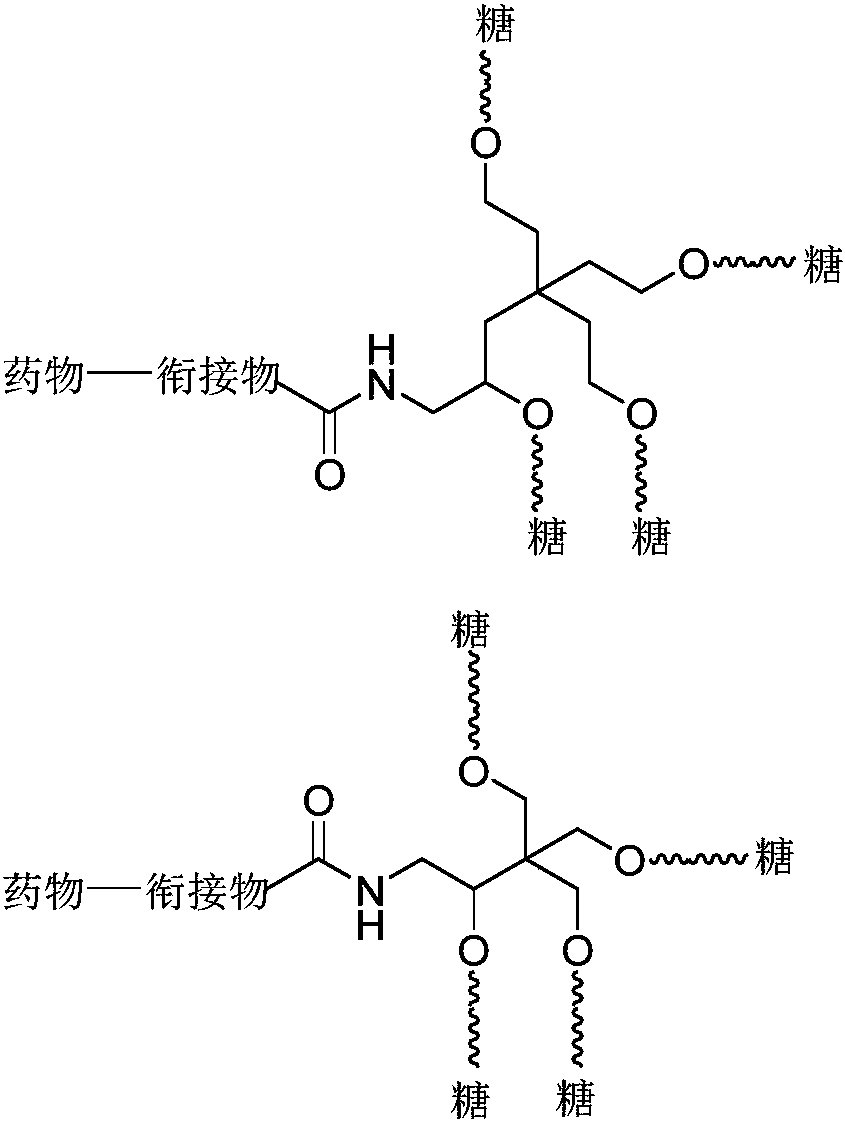
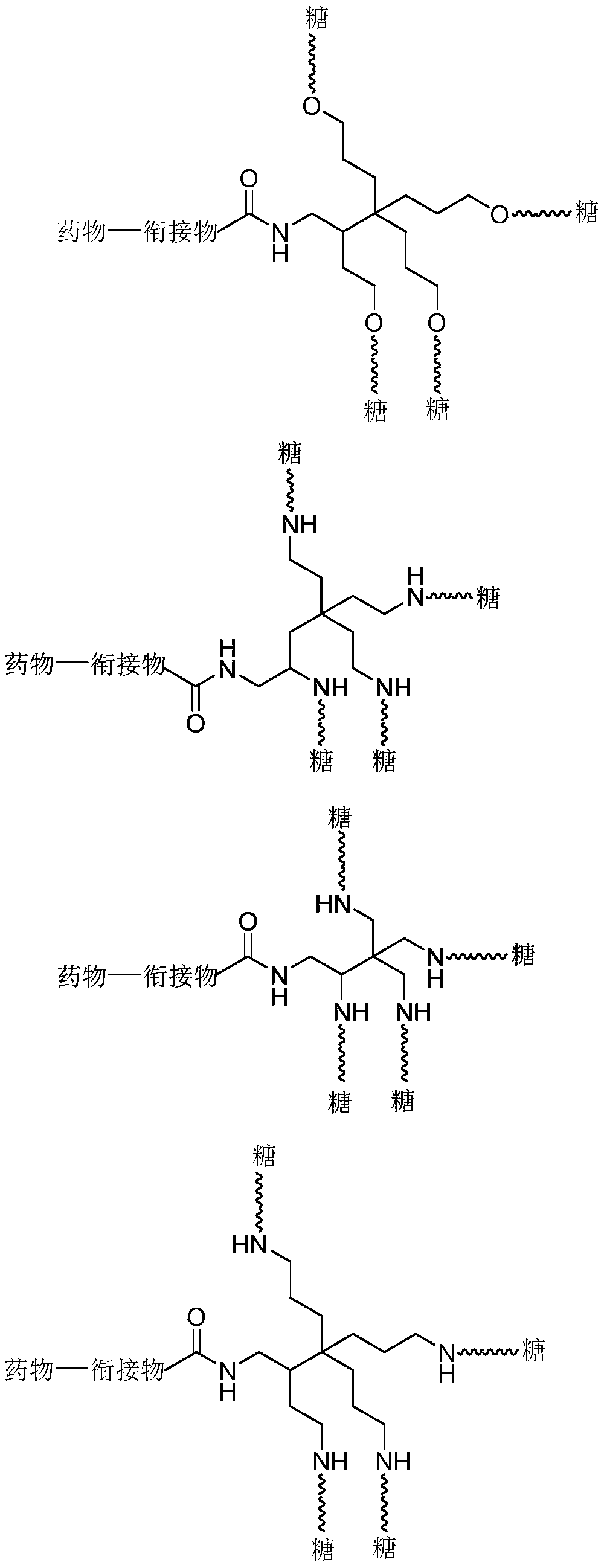
Novel liver targeting drug carrier
A liver-targeting and drug-based technology, applied in the field of new liver-targeting drug carriers, can solve problems such as large limitations, narrow therapeutic index, and insignificant targeting, and achieve stability, high biological stability, and high efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Acetylation of D-galactosamine
[0047] 400 milliliters of anhydrous pyridine (Py) was added 90 grams of acetic anhydride (Ac 2 O) (300 ml) suspension, then add 4.2 g of 4-dimethylaminopyridine (DMAP) and 65 ml of triethylamine (Et 3 N), the reaction mixture was stirred overnight, and the reaction process was monitored by TLC until the raw materials were substantially reacted, and the precipitated solid was filtered, washed with toluene and water respectively, and dried in vacuo to obtain 145 grams of fully acetylated D-galactosamine.
Embodiment 2
[0048] Example 2: Activation of peracetyl-D-galactosamine
[0049] 100 g of peracetyl-D-galactosamine was suspended in 1 liter of anhydrous dichloroethane (DCE) under nitrogen protection, and 56 ml of trimethylsilyl trifluoromethanesulfonate (TMSOTf) was The reaction suspension was slowly added dropwise in the atmosphere, and then the reaction mixture was stirred at room temperature of 25°C for 16 hours. The reaction mixture was carefully transferred to 3 liters of vigorously stirred ice-water suspension containing 15 wt% sodium bicarbonate and continued to stir for 1 hour, the organic phase was separated, and the aqueous phase was extracted twice with dichloromethane (DCM). The organic phases were combined and dried over anhydrous magnesium sulfate, filtered and concentrated to dryness to yield 91 g of product.
Embodiment 3
[0050] Example 3: Protection of glycosyl-linked carboxylate
[0051] 50 grams of the above-mentioned product of Example 2 and 50 grams of benzyl 4-hydroxybutyrate were dissolved in 300 milliliters of anhydrous dichloroethane (DCE), added 50 grams of molecular sieves and stirred for 20 minutes, then added 7 milliliters of trifluoroform Trimethylsilylsulfonate (TMSOTf) and stirred at 25°C for 16 hours. The reaction solution was poured into 600 ml of sodium bicarbonate solution mixed with ice water, stirred for 1 hour, the organic phase was separated, the aqueous phase was extracted twice with DCM, the organic phases were combined and dried with anhydrous magnesium sulfate, filtered and concentrated to obtain a crude product, The crude product was purified by column chromatography to obtain 73 g of light yellow oily liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com