Method for loading medicines on tumor targeted amorphous calcium phosphate fluorescence nanocomposite materials
An amorphous calcium phosphate, fluorescent nanomaterial technology, applied in nanomedicine, antitumor medicine, nanotechnology and other directions, can solve the problems of tissue and organ damage, unsatisfactory curative effect, damage and other problems, achieve good biocompatibility, The effect of realizing the integration of diagnosis and treatment and good biological imaging effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
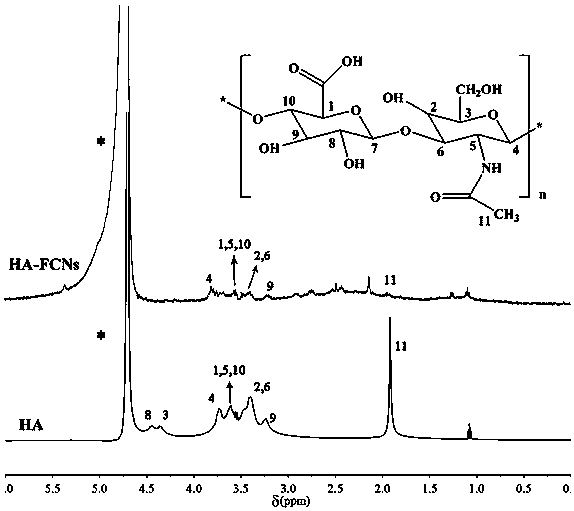
[0035] This example provides a method for tumor-targeting amorphous calcium phosphate fluorescent nanocomposite HA-FCNs / ACP, which includes the following steps: firstly synthesize partially carbonized hyaluronic acid (HA-FCNs) by high temperature dehydration, and prepare the HA -FCNs were added to the preparation process of ACP, and the amorphous calcium phosphate fluorescent composite material (HA-FCNs / ACP) was synthesized by co-precipitation method. Specific steps are as follows:
[0036] (1) Dissolve 0.3 g of hyaluronic acid in 10 mL of deionized water, add 10 mL of concentrated sulfuric acid into the aqueous solution of hyaluronic acid under the condition of stirring in an oil bath at 230 °C, stir for 1.5 min, and dialyze with a molecular weight of 500 Da The bag was dialyzed for three days and freeze-dried to obtain HA-FCNs fluorescent material.
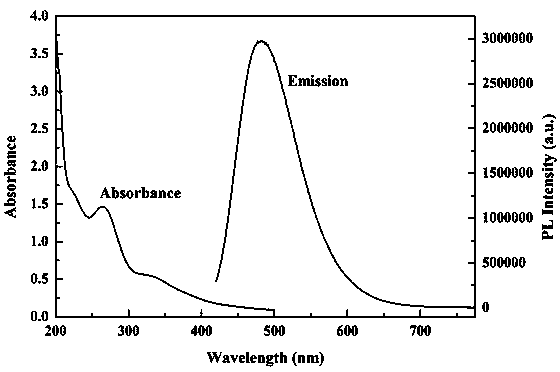
[0037] The prepared HA-FCNs nanoparticles were tested by fluorescence emission spectrum and ultraviolet spectrum ( figure 1 ...
Embodiment 2
[0042] (1) Dissolve 0.4 g of hyaluronic acid in 10 mL of deionized water, add 15 mL of concentrated sulfuric acid into the aqueous solution of hyaluronic acid under the condition of stirring in an oil bath at 230 °C, stir for 2 min, and dialyze with a molecular weight of 2000 Da The bag was dialyzed for three days and freeze-dried to obtain HA-FCNs fluorescent material.
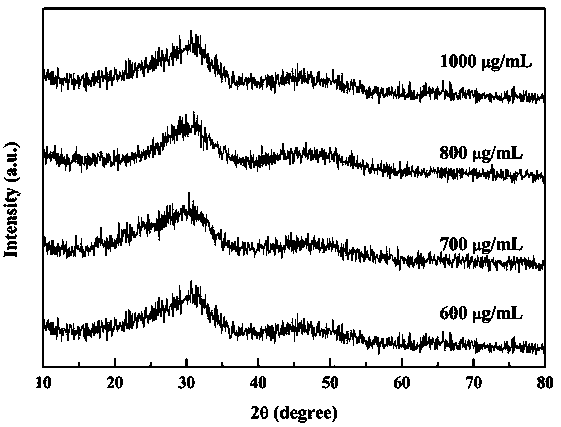
[0043] (2) Prepare 0.034 mol / L 29 mL calcium nitrate aqueous solution, 0.024 mol / L 29 mL diammonium hydrogen phosphate aqueous solution, 1 mol / L 50 mL NaOH solution, 700 μg / mL 30 mL HA-FCNs aqueous solution and 0.6 mg / L mL 10 mL DOX aqueous solution, in which, ultrasonically disperse DOX aqueous solution (ultrasonic conditions: probe ultrasonic power 500 W, ultrasonic 2 s, intermittent 3 s). The HA-FCNs aqueous solution was added dropwise to the calcium nitrate solution at 35 ºC, and after blending and stirring for 1.5 h, the DOX aqueous solution was added dropwise to the above mixture, and stirred for 1 h. ...
Embodiment 3
[0046] (1) Dissolve 0.2 g of hyaluronic acid in 10 mL of deionized water, add 15 mL of concentrated sulfuric acid into the aqueous solution of hyaluronic acid under the condition of stirring in an oil bath at 240 °C, stir for 1 min, and dialyze with a molecular weight of 3500 Da The bag was dialyzed for three days and freeze-dried to obtain HA-FCNs fluorescent material.
[0047] (2) Preparation of Cur-loaded composite materials: 0.034 mol / L 29 mL calcium nitrate aqueous solution, 0.024 mol / L 29 mL diammonium hydrogen phosphate aqueous solution, 1 mol / L 50 mL NaOH solution, 800 μg / mL 30 mL HA-FCNs aqueous solution and 5 mg / mL 10 mL Cur aqueous solution, in which the Cur aqueous solution was dispersed ultrasonically (ultrasonic conditions: probe ultrasonic power 400 W, ultrasonic 3 s, intermittent 3 s). The HA-FCNs aqueous solution was added dropwise to the calcium nitrate solution at 25 ºC, and after blending and stirring for 1 h, the Cur aqueous solution was added dropwise to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com