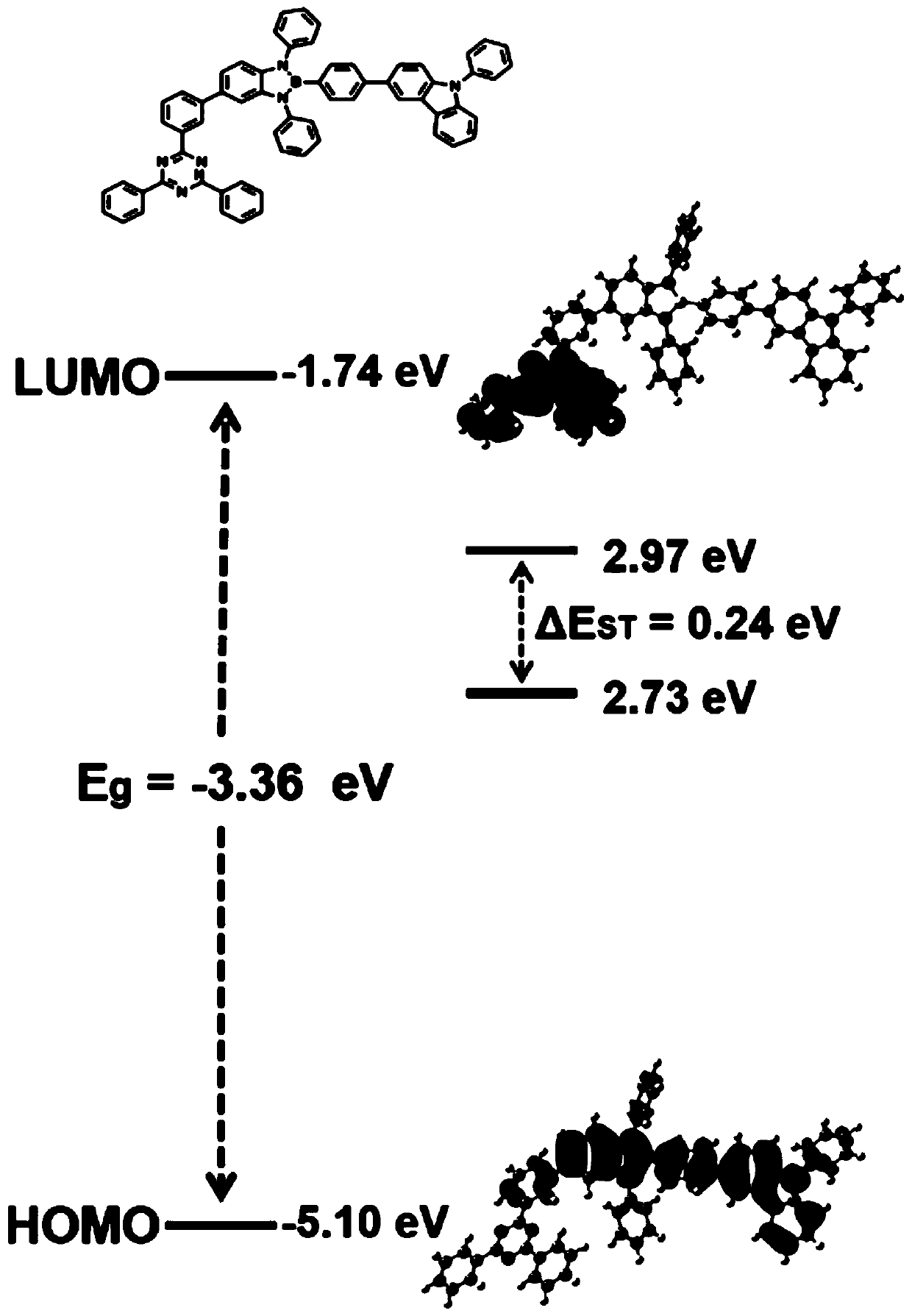
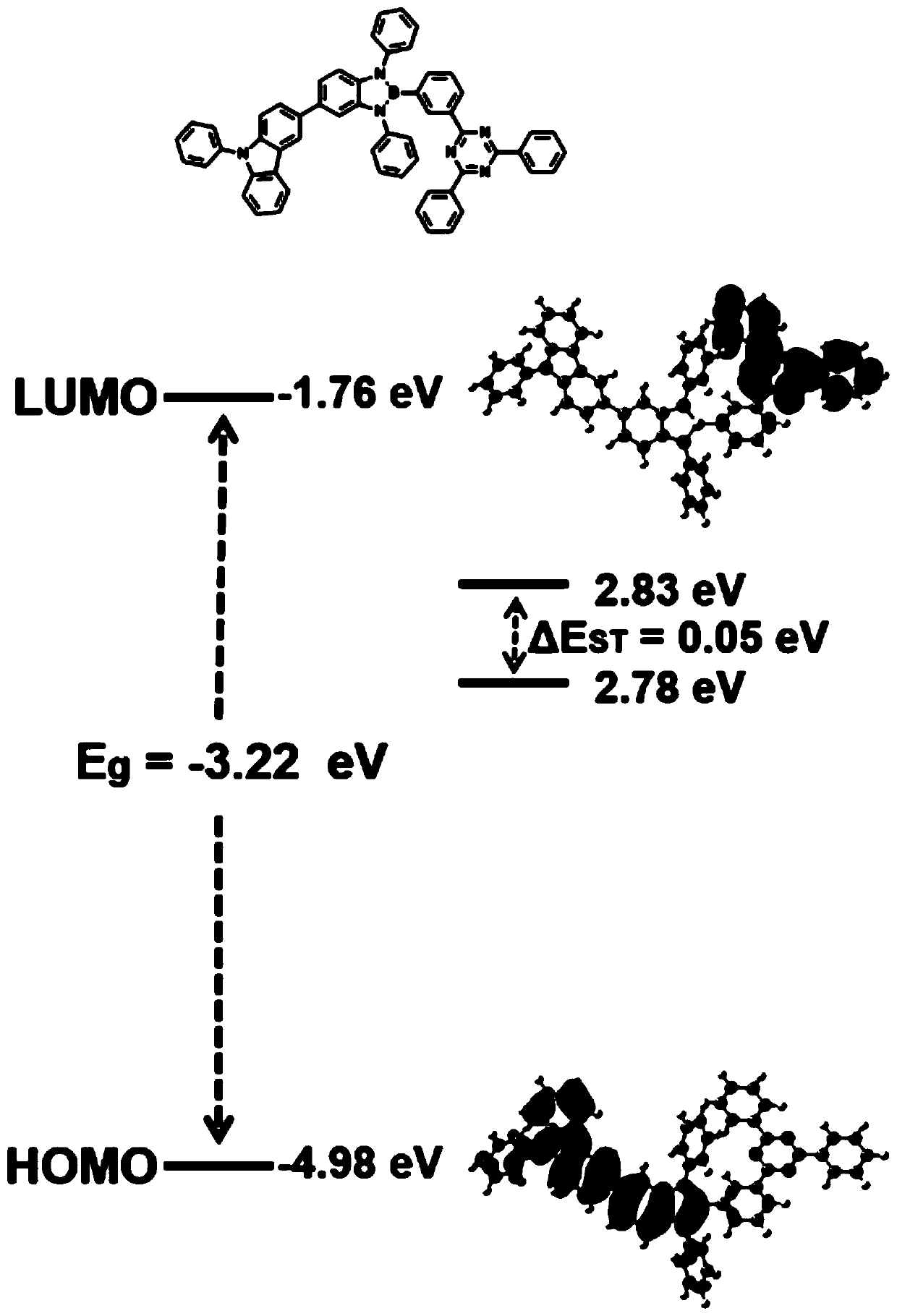
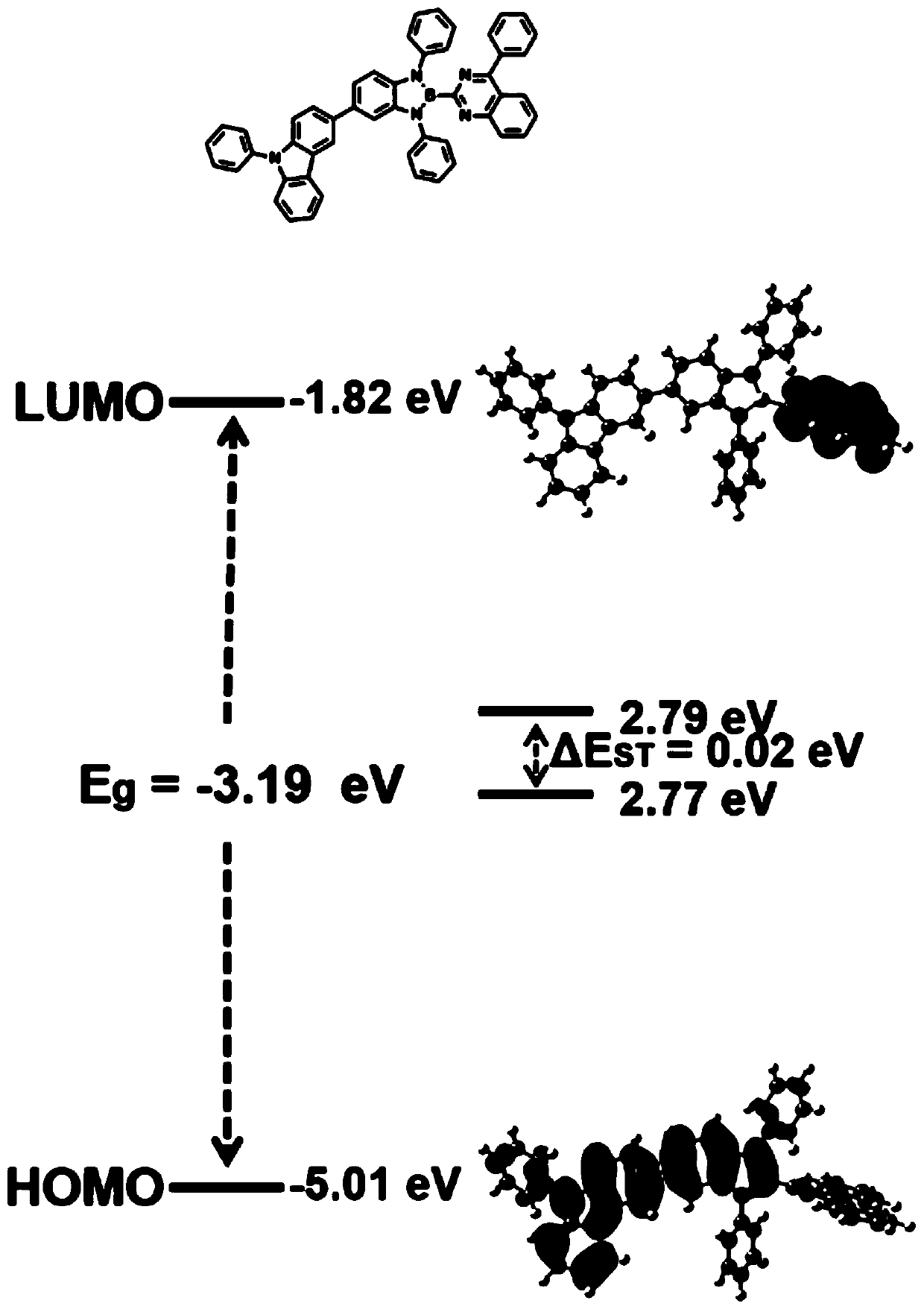
Boron-nitrogen heteropolyaromatic ring compound and application thereof
An aromatic ring compound and heteropoly technology, which is applied in the fields of compounds containing Group 3/13 elements of the periodic table, organic chemistry, chemical instruments and methods, etc., can solve the problems of low performance and luminous efficiency of OEL devices, energy level, charge The mobility stability is difficult to achieve a good unity, and the glass transition temperature is low.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] This embodiment provides a boron azapolyaromatic ring compound, and the synthesis route of compound C-1 is as follows:
[0062]
[0063] The preparation method of compound C-1 specifically includes the following steps:
[0064] 1) Add 4-bromo-o-phenylenediamine (37.4g, 0.20mol), bromobenzene (66.0g, 0.42mol), toluene (500mL), and sodium tert-butoxide (84.6g, 0.88mol) in a three-necked flask. Add tri-tert-butyl phosphine (2.0mmol) under nitrogen protection, Pd 2 (dba) 3 .CHCl 3 (1.04g, 1.0mmol), the resulting solution was refluxed at 110°C for 8 hours, cooled to room temperature, filtered to obtain the filtrate and filter cake, the resulting filter cake was washed three times with toluene (100mL*3), and the washing solution and the filtrate were combined to obtain organic The organic phase was spin-dried, the solid obtained was purified with a silica gel column (eluent PE:EA=20:1 to 5:1), the eluent was spin-dried to obtain the product C-1-C (51.3g, yield 75.6 %).
[0065] 2)...
Embodiment 2
[0070] This embodiment provides an azapolyaromatic ring compound, and the synthesis route of compound C-2 is as follows:
[0071]
[0072] The preparation method of compound C-2 specifically includes the following steps:
[0073] 1) Add C-1-F (57.4g, 0.20mol), p-dibromobenzene (51.9g, 0.22mol), toluene (500mL), sodium tert-butoxide (38.4g, 0.40mol) in a three-necked flask, Add dppf (1,1'-bis(diphenylphosphine) ferrocene, 2.0mmol), Pd under the protection of nitrogen 2 (dba) 3 .CHCl 3 (1.04g, 1.0mmol), the resulting solution was refluxed at 110°C for 8 hours, cooled to room temperature, filtered to obtain the filtrate and filter cake, the resulting filter cake was washed three times with toluene (100mL*3), and the washing solution and the filtrate were combined to obtain organic The organic phase was spin-dried, the solid obtained was purified with a silica gel column (eluent PE:EA=30:1 to 15:1), the eluent was spin-dried to obtain the product C-2-A (68.2g, yield 85.6 %).
[0074] 2...
Embodiment 3
[0079] This embodiment provides an azapolyaromatic ring compound, and the synthesis route of compound C-4 is as follows:
[0080]
[0081] The preparation method of compound C-4 specifically includes the following steps:
[0082] 1) Add C-1-C (67.8g, 0.20mol) to toluene (400mL), add phenylboronic acid (24.4g, 0.20mol), the resulting solution is refluxed at 110°C for 3 hours, cooled, and the precipitated solid is filtered. The cake was washed with a small amount of toluene to obtain a crude product, and the crude product was vacuum-baked at 80°C for 8 hours to obtain the product C-4-A (79.0 g, yield 93.1%).
[0083] 2) Dissolve C-4-A (42.5g, 0.10mol) in a mixed solution of tetrahydrofuran (300mL) and water (75mL), add C-1-F (28.7g, 0.10mol), add potassium carbonate (27.6g) , 0.20mol), tetrakis(triphenylphosphine)palladium (500mg) was added under nitrogen protection, the resulting solution was refluxed for 8 hours, cooled to room temperature, and extracted with ethyl acetate (200mL*2)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com