A kind of preparation method of 24-epibrassin intermediate (22e, 24r)-3α, 5-ring-5α-ergosta-7,22-dien-6-one
A technology for intermediates and surface Brassica, applied in the field of preparation of plant growth regulators, can solve the problems of low total yield, high operator requirements, environmental pollution, etc., and achieves the effects of fast reaction rate and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
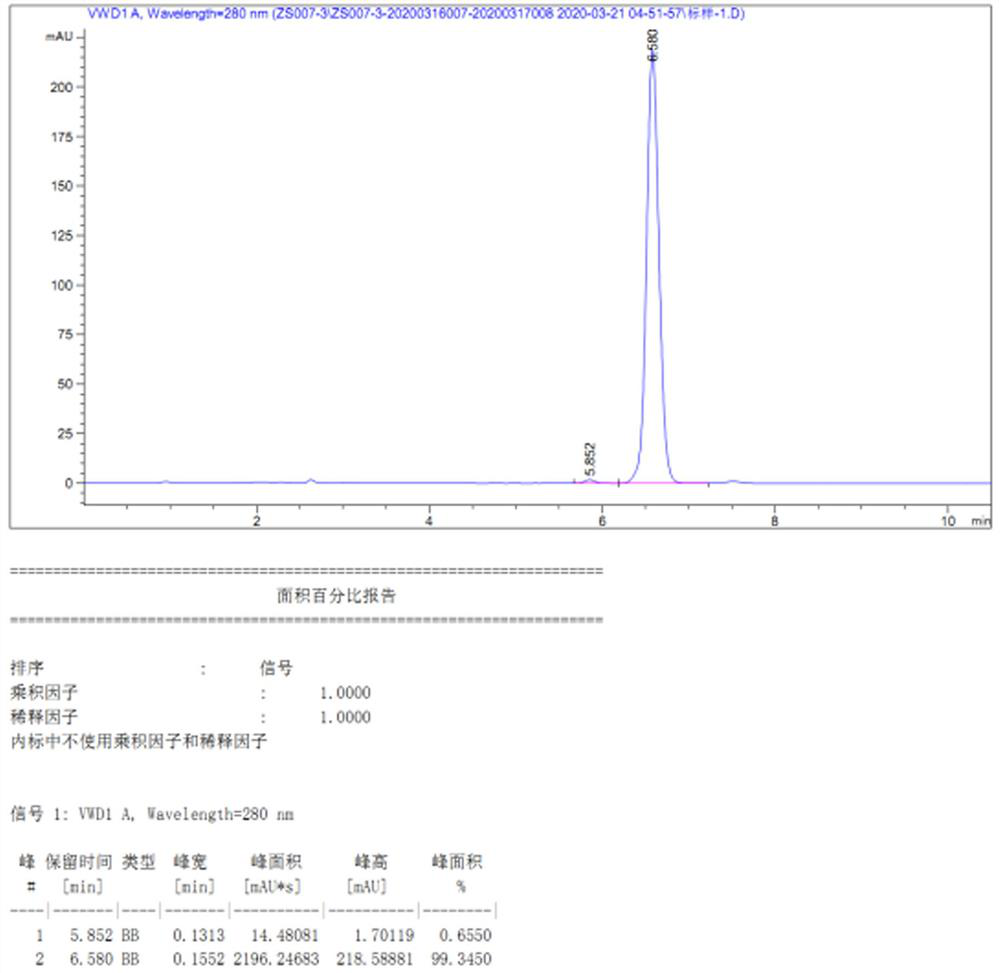
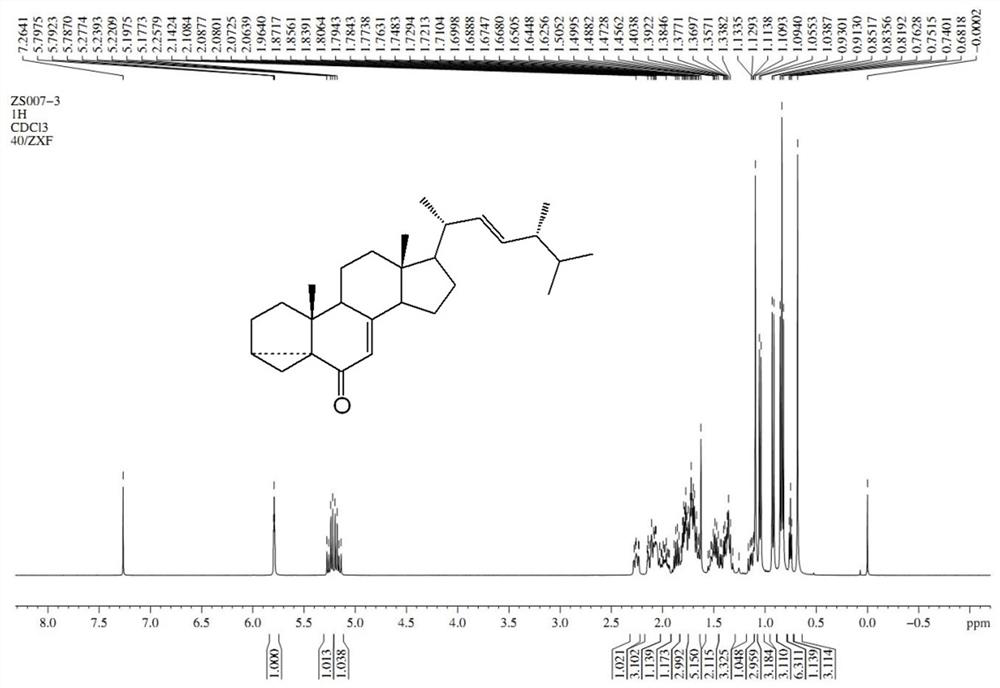
Examples
Embodiment 1
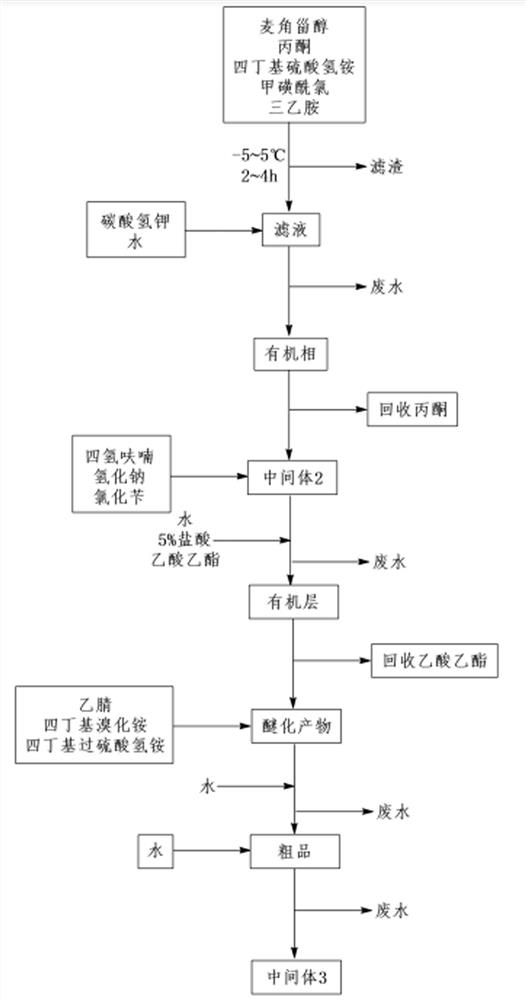
[0045] A preparation method of 24-epibrassin intermediate (22E, 24R)-3α, 5-ring-5α-ergosta-7,22-dien-6-one, comprising the following steps:
[0046] 1) Add 100.0 g of ergosterol, 800.0 g of acetone and 4.3 g of tetrabutylammonium bisulfate to the reaction flask, stir for 0.5 h, cool down to -5~5°C with ice-cold brine, add 29.0 g of methanesulfonyl chloride, and Add 25.5g of triethylamine dropwise at 5-5°C for 0.5h. After the dropwise addition, continue the reaction at -5-5°C for 4h. After the reaction, intermediate 1 is obtained. Filter the reaction solution to obtain a white solid and pale yellow filtrate containing intermediate 1;
[0047] 2) Add 200 g of water and 32.8 g of potassium bicarbonate to the light yellow filtrate containing intermediate 1 obtained in step 1), stir and heat up to 50° C. and keep the temperature for 5 hours. After the reaction, stop stirring under heat preservation, and separate the phases. The lower aqueous phase was separated, and the upper orga...
Embodiment 2
[0053] A preparation method of 24-epibrassin intermediate (22E, 24R)-3α, 5-ring-5α-ergosta-7,22-dien-6-one, comprising the following steps:
[0054] 1) Add 100.0 g of ergosterol, 900.0 g of acetone and 8.6 g of tetrabutylammonium bisulfate to the reaction flask, stir for 0.5 h, cool down to -5~5°C with ice-cold brine, add 58.0 g of methanesulfonyl chloride, and Add 51.0g of triethylamine dropwise at 5~5°C for 0.5h. After the dropwise addition, continue the reaction at -5~5°C for 3h. After the reaction, Intermediate 1 is obtained. Filter the reaction solution to obtain a white solid and pale yellow filtrate containing intermediate 1;
[0055] 2) Add 150 g of water and 50.0 g of potassium bicarbonate to the light yellow filtrate containing intermediate 1 obtained in step 1), stir and heat up to 55° C. and keep the temperature for 3 hours. After the reaction, stop stirring under heat preservation, and separate the phases. The lower aqueous phase was separated, and the upper orga...
Embodiment 3
[0060] A preparation method of 24-epibrassin intermediate (22E, 24R)-3α, 5-ring-5α-ergosta-7,22-dien-6-one, comprising the following steps:
[0061] 1) Add 100.0 g of ergosterol, 1000.0 g of acetone and 43.0 g of tetrabutylammonium bisulfate to the reaction flask, stir for 0.5 h, cool down to -5~5°C with ice-cold brine, add 87.0 g of methanesulfonyl chloride, and 76.5g of triethylamine was added dropwise at 5-5°C for 0.5h. After the dropwise addition, the reaction was continued at -5-5°C for 2h. After the reaction, Intermediate 1 was obtained. The reaction solution was filtered to obtain a white solid and pale yellow filtrate containing intermediate 1;
[0062] 2) Add 100 g of water and 75.0 g of potassium bicarbonate to the light yellow filtrate containing intermediate 1 obtained in step 1), stir and heat up to 60° C. and keep the temperature for 2 hours. After the reaction, stop stirring under heat preservation, and separate the phases. The lower aqueous phase was separated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com