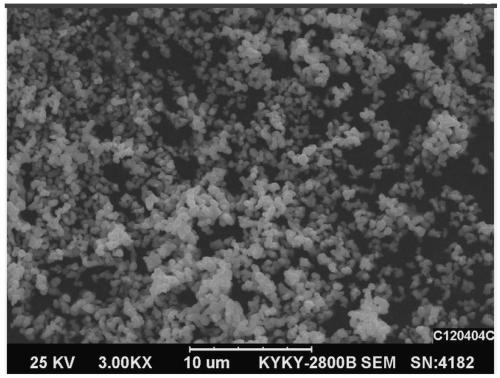
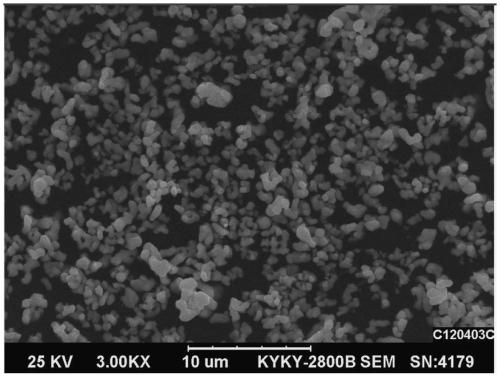
Preparation method of phosphorus-doped lithium nickel cobalt ferrite
A technology of cobalt lithium ferrite and phosphorus doping, applied in chemical instruments and methods, nickel compounds, lithium batteries, etc., can solve problems such as structural instability, and achieve high density, large BET, and uniform mixing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A preparation method for phosphorus-doped nickel-cobalt lithium ferrite, which comprises the following steps:
[0046] 1) Add nickel-cobalt salt into pure water and stir to dissolve to obtain a nickel-cobalt solution. Add ammonium bicarbonate solution to maintain the pH of the process at 7 and the temperature at 35°C. After the addition, the temperature is raised to 50°C. After 15 minutes of reaction, stop React, filter and wash to obtain nickel-cobalt carbonate precipitation;
[0047] 2) adding the nickel-cobalt carbonate precipitate to the phosphoric acid solution, stirring and reacting for 15 minutes, then stopping the reaction to obtain nickel-cobalt precipitate doped with phosphate;
[0048] 3) Add the phosphate-doped nickel-cobalt precipitate obtained in step (2) into polyethylene glycol solution and lithium bicarbonate solution to stir and slurry, then after spray-drying, pass the spray-dried material into an atmosphere with an oxygen volume concentration of 95.8...
Embodiment 2
[0064] A preparation method for phosphorus-doped nickel-cobalt lithium ferrite, which comprises the following steps:
[0065] 1) Add nickel-cobalt salt into pure water and stir to dissolve to obtain a nickel-cobalt solution. Add ammonium bicarbonate solution to maintain the pH of the process at 7.5 and the temperature at 45°C. After the addition, the temperature is raised to 60°C. After 25 minutes of reaction, stop React, filter and wash to obtain nickel-cobalt carbonate precipitation;
[0066] 2) adding the nickel-cobalt carbonate precipitate to the phosphoric acid solution, stirring and reacting for 30 minutes, then stopping the reaction to obtain a nickel-cobalt precipitate doped with phosphate;
[0067] 3) Add the phosphate-doped nickel-cobalt precipitate obtained in step (2) into polyethylene glycol solution and lithium bicarbonate solution to stir and slurry, then after spray-drying, pass the spray-dried material into an atmosphere with an oxygen volume concentration of ...
Embodiment 3
[0083] A preparation method for phosphorus-doped nickel-cobalt lithium ferrite, which comprises the following steps:
[0084] 1) Add nickel-cobalt salt into pure water and stir to dissolve to obtain a nickel-cobalt solution. Add ammonium bicarbonate solution to maintain the pH of the process at 7.3 and the temperature at 40°C. After the addition, the temperature is raised to 55°C. After 20 minutes of reaction, stop React, filter and wash to obtain nickel-cobalt carbonate precipitation;
[0085] 2) adding the nickel-cobalt carbonate precipitate into the phosphoric acid solution, stirring and reacting for 25 minutes, then stopping the reaction to obtain a nickel-cobalt precipitate doped with phosphate;
[0086] 3) Add the phosphate-doped nickel-cobalt precipitate obtained in step (2) into polyethylene glycol solution and lithium bicarbonate solution to stir and slurry, then after spray-drying, pass the spray-dried material into an atmosphere with an oxygen volume concentration o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Primary particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com