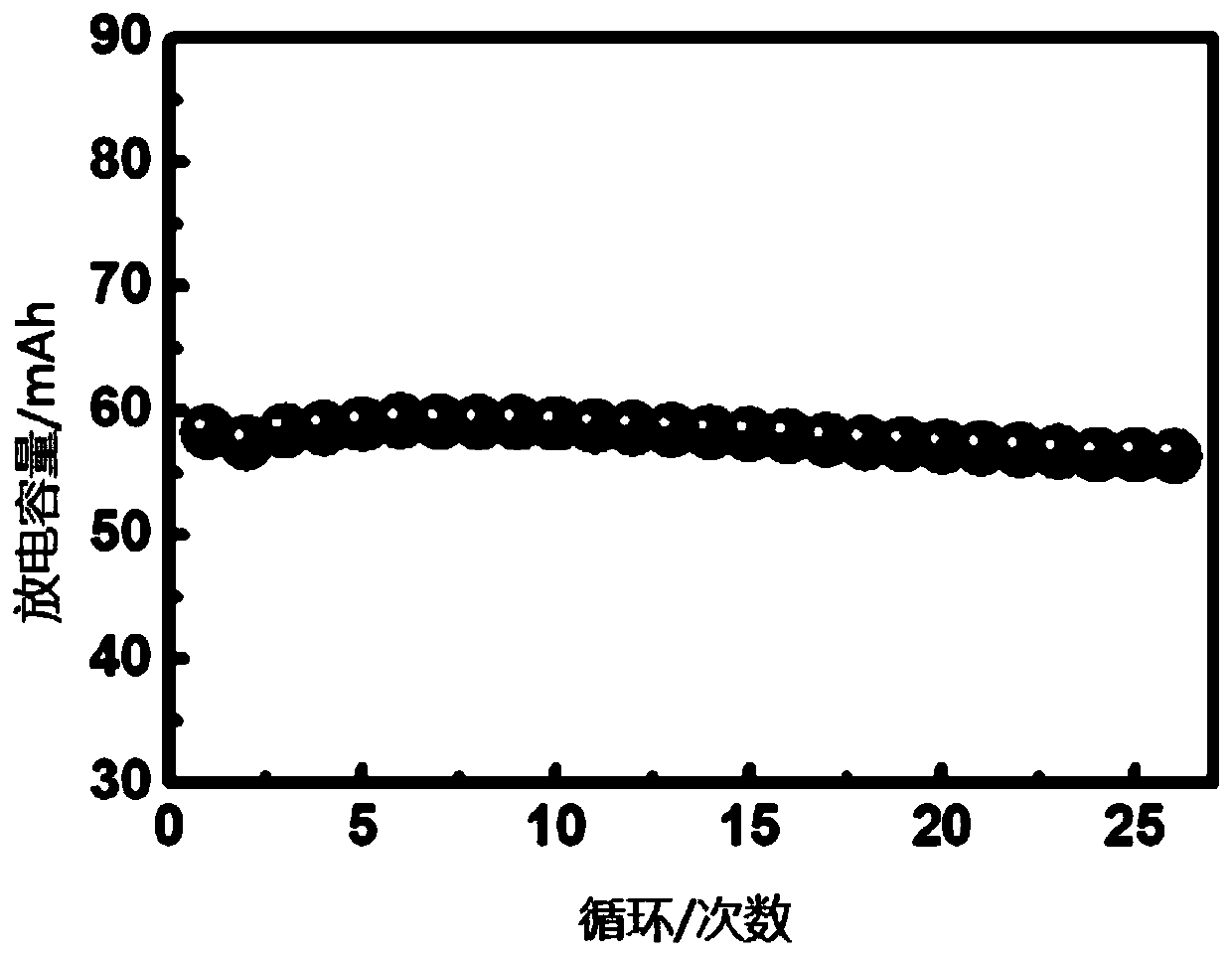
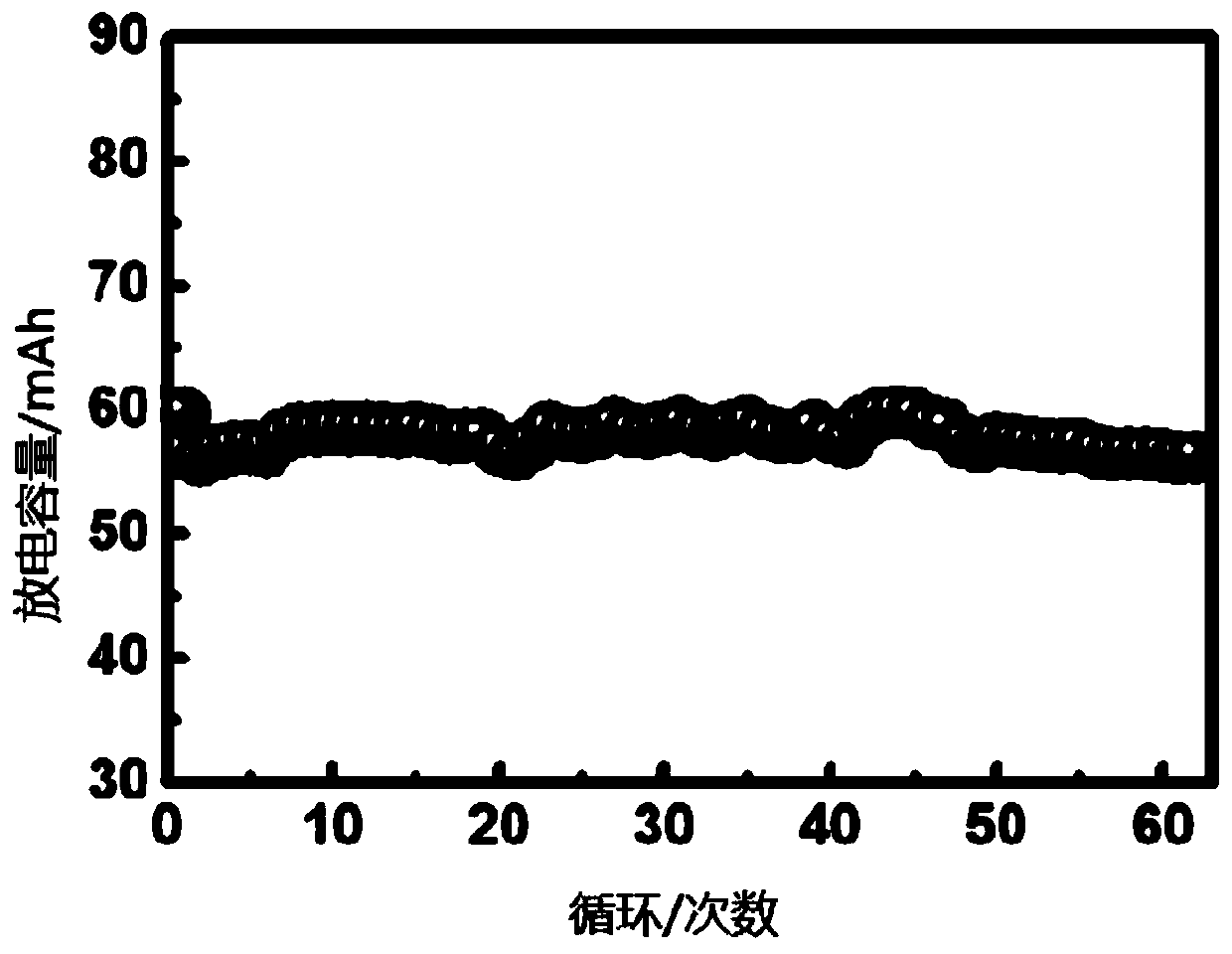
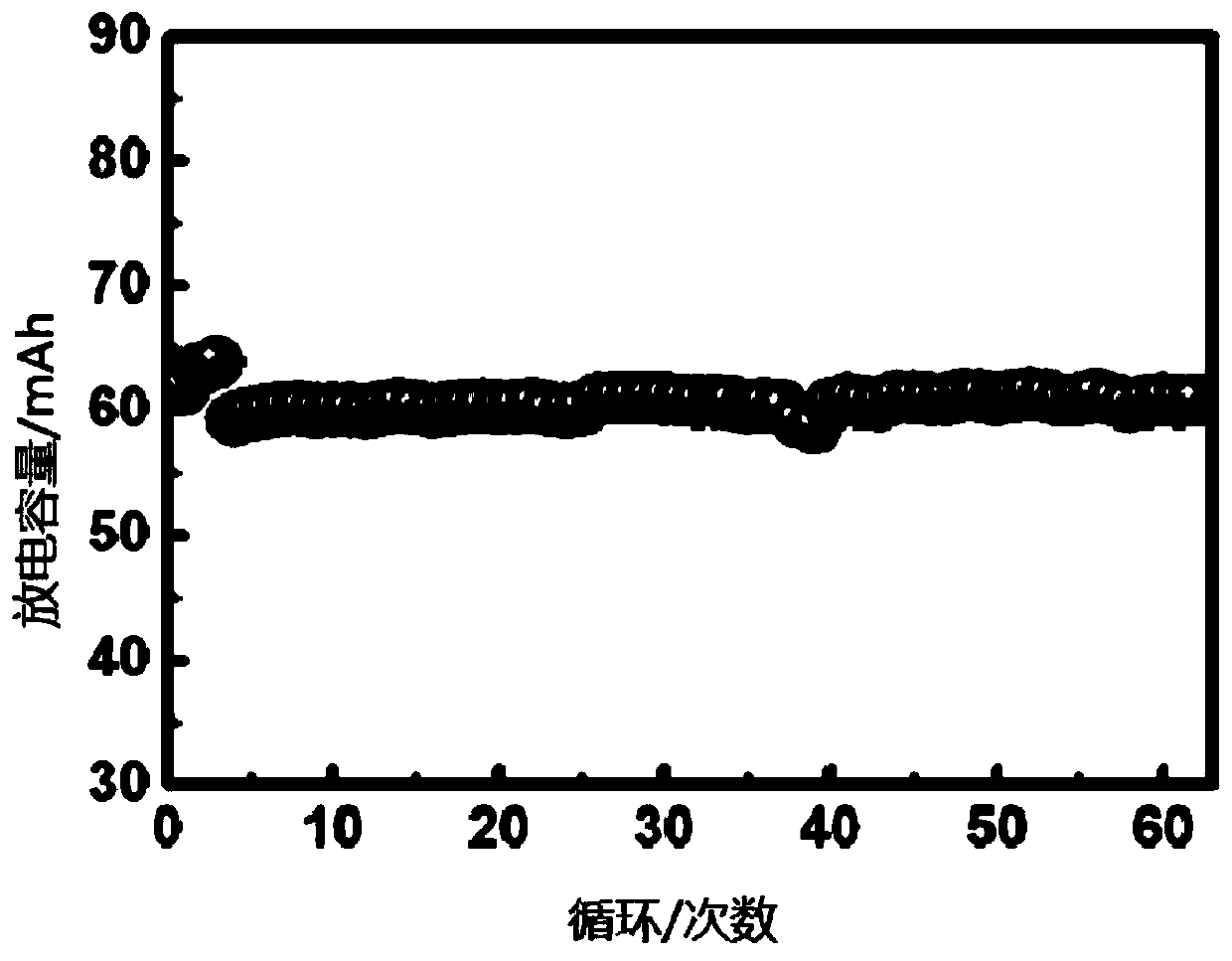
Solid-state polymer electrolyte, solid-state battery comprising same and preparation method of solid-state battery
A technology of solid polymers and amorphous polymers, applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, secondary batteries, etc., can solve the problem of poor interface performance, poor effect of inhibiting lithium dendrites, and toxicity of polymer electrolytes Application and other issues to achieve the effect of improving antioxidant capacity, broadening the electrochemical window, and promoting chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a glove box filled with argon, 10.8g (0.1mol) DAMN (denoted as monomer A1 ) Dissolve in 20ml of anhydrous acetonitrile, add 2mmol of initiator 1-chloro-1-phenylethane, stir well, then add 2mmol of catalyst CuCl and 4mmol of ligand bipyridine (bpy), and mix well, The solution A1 was prepared. The solution A1 was heated to 80°C in an oil bath. After 12 hours of reaction and polymerization, the reaction was terminated, and then the solution was diluted with THF and passed through Al 2 O 3 The column was purified and washed with anhydrous ether to obtain a purified partially polymerized PDAMN, which was denoted as segment C1 and had a molecular weight of 18,000.
[0025] 0.54g PDAMN (segment C1) was mixed with 5mmol CuCl, 5mmol bpy and dissolved in anhydrous acetonitrile, then 2.94g methoxy polyethylene glycol methacrylate (denoted as monomer B1, M n =980, n=20), the reaction temperature was increased to 110°C, the reaction was terminated after 6 hours of reaction, and after...
Embodiment 2
[0028] In a glove box filled with argon, weigh 3g of A-B obtained in the manner in Example 1 in the reaction vessel 100 The type of macroinitiator D1 is dissolved in anhydrous acetonitrile, and then 0.1 mmol of catalyst CuBr, 0.1 mmol of ligand bipyridine (bpy) and 0.0108 g of monomer A1 are added, and mixed uniformly to prepare solution B1. Place solution B1 in The temperature was raised to 110℃ in the oil bath, the reaction was terminated after 5 hours of polymerization, and after purification and solvent removal, AB was obtained 100 -A type PDAMN-b-P(PEGMA)-b-PDAMN triblock copolymer, denoted as macroinitiator D2, M n Is 40,000.
[0029] Take 1M LiPF 6 Add 1g of macroinitiator D2, 0.0025mmol of CuCl, 0.0025mmol of bpy and 2.45g of monomer B, and mix well to obtain solution B2. The mixed solution B2 is injected into a lithium-ion battery with a PP diaphragm as a support material, and the battery is placed in a 60°C constant temperature oven for 48 hours to polymerize, so that th...
Embodiment 3
[0031] In a glove box filled with argon gas, 14.4g (0.1mol) monomer A2( ) Was dissolved in 25ml of anhydrous acetonitrile, and then 2mmol of initiator dichloromethane was added, and stirred evenly, then 2mmol of catalyst CuBr and 4mmol of tris(2-methylamino)ethylamine (Me 6 TREN) and mixed uniformly to prepare solution A2. The solution A2 was heated to 100°C in an oil bath. After 8 hours of reaction and polymerization, the reaction was terminated. After purification, solvent removal, and washing with anhydrous ether, a refined macromolecular initiator was obtained. Agent C2, the molecular weight is 16000.
[0032] 0.32g macroinitiator C2, 2mmol CuBr, 2mmol Me 6 TREN was mixed and dissolved in anhydrous acetonitrile, and 4g polyethylene glycol methacrylate (denoted as monomer B2, M n =1000, n=600, ), the reaction temperature was raised to 100°C, and the reaction was terminated after 6 hours of reaction. After purification and solvent removal, A-B 200 Type diblock copolymer, denote...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com