Ni-Fe catalyst, and preparation method and application thereof
A catalyst, ni-fe technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of high production cost of electrocatalysts, difficult long-term high-efficiency electrolysis , the problem of high overpotential, to achieve the effect of being beneficial to industrial application, the preparation method is simple and efficient, and the performance is excellent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
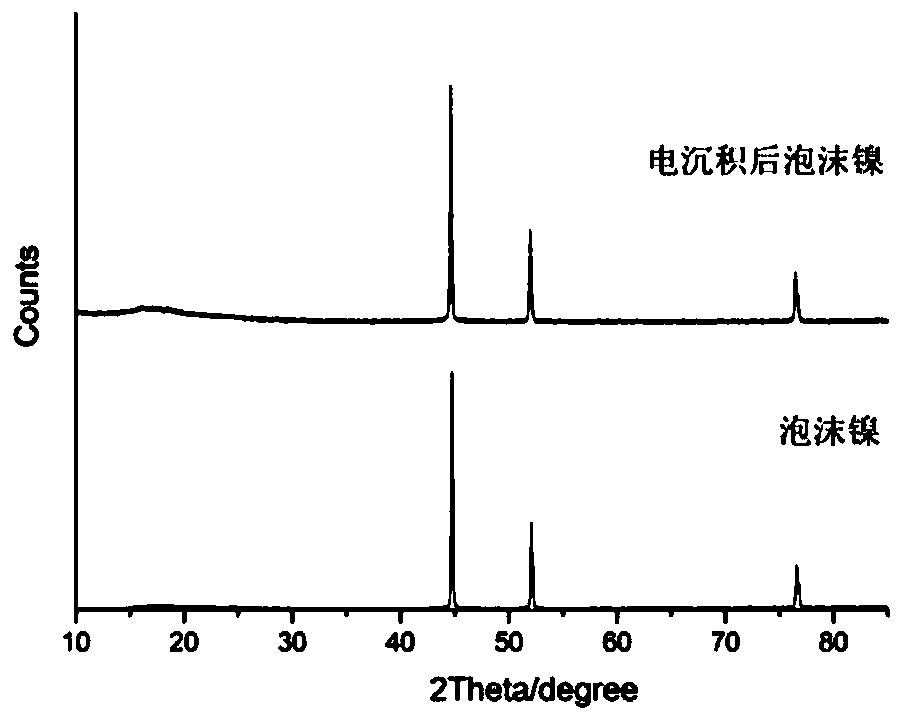
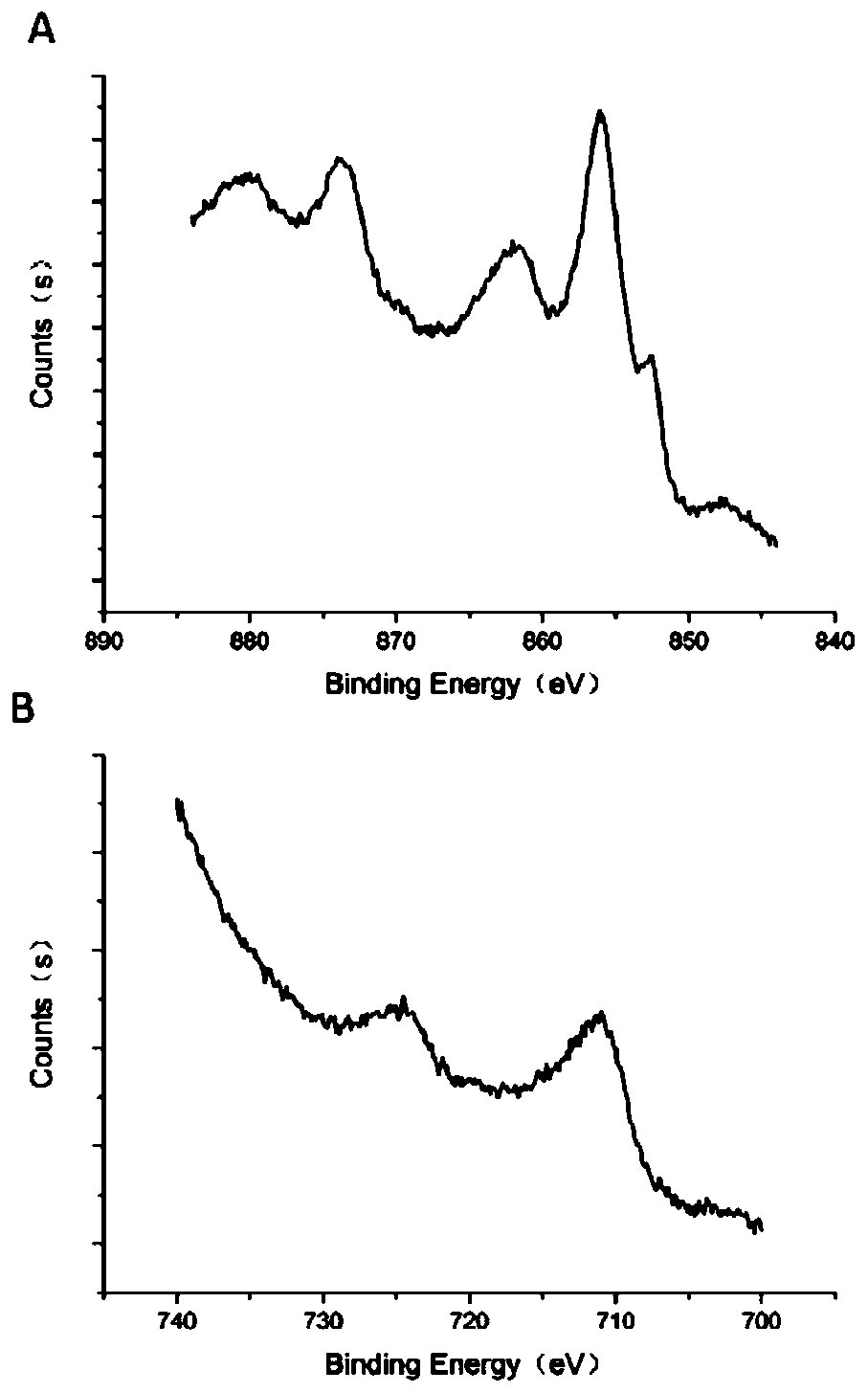
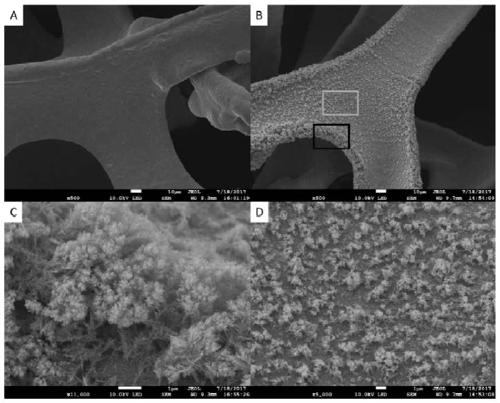
Image
Examples
Embodiment 1
[0027] The present embodiment provides a kind of preparation method of Ni-Fe catalyst, and concrete steps are as follows:
[0028] Step 1: Cut the nickel foam substrate and carbon paper into 0.5cm×3.0cm, wash them with acetone, ethanol, and deionized water for 30 minutes, and dry them at 60°C for later use.
[0029] Step 2: Prepare ferric nitrate and nickel nitrate solutions of 309mmol / L respectively for later use.
[0030] Step 3: Add 0.7ml of the ferric nitrate and nickel nitrate solutions of the above concentrations into 100ml of ethylene glycol, and then add 0.7ml of deionized water and 0.11g of ammonium fluoride; ultrasonicate for a while to form a uniform solution.
[0031] Step 4: Use clean nickel foam as the cathode, and carbon paper as the anode, the two are parallel to each other about 2cm apart, and the working area is 2.0cm×0.5cm. Put it into the precursor solution that has been preheated to 40°C, and let it stand for a while.
[0032] Step 5: Keep the cathode an...
Embodiment 2
[0040] Example 1 was repeated.
[0041] The results show that the current density of electrolyzing water to produce oxygen reaches 10mA / cm 2 The overpotential is 230mV, 100mA / cm 2 The overpotential is 266mV, 300mA / cm 2 The overpotential of 289mV.
Embodiment 3
[0043] Example 1 was repeated.
[0044] The results show that the current density of electrolyzing water to produce oxygen reaches 10mA / cm 2 The overpotential is 223mV, 100mA / cm 2 The overpotential is 255mV, 300mA / cm 2 The overpotential of 261mV.
[0045] Examples 2 and 3 show that the method has good repeatability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com