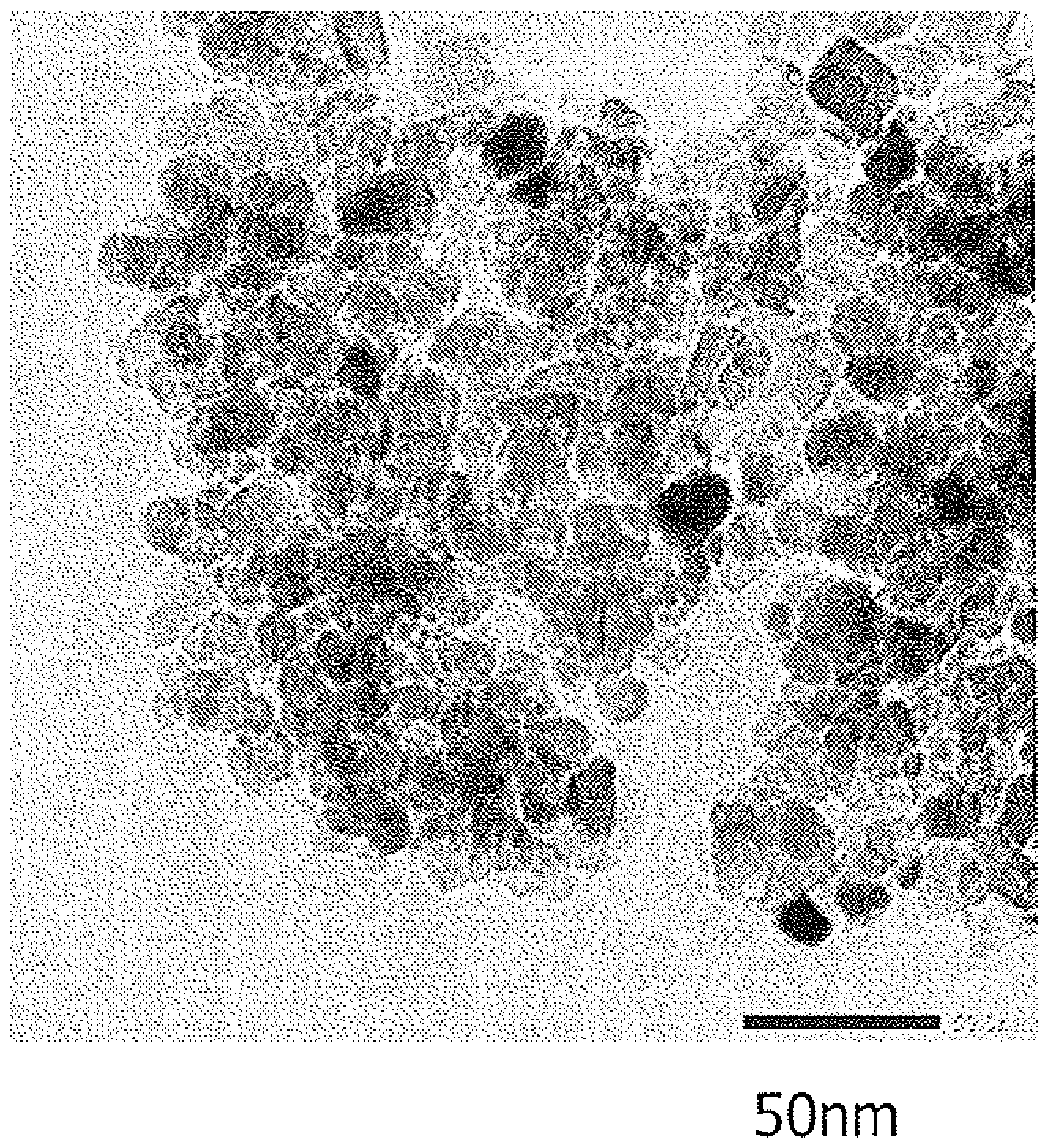
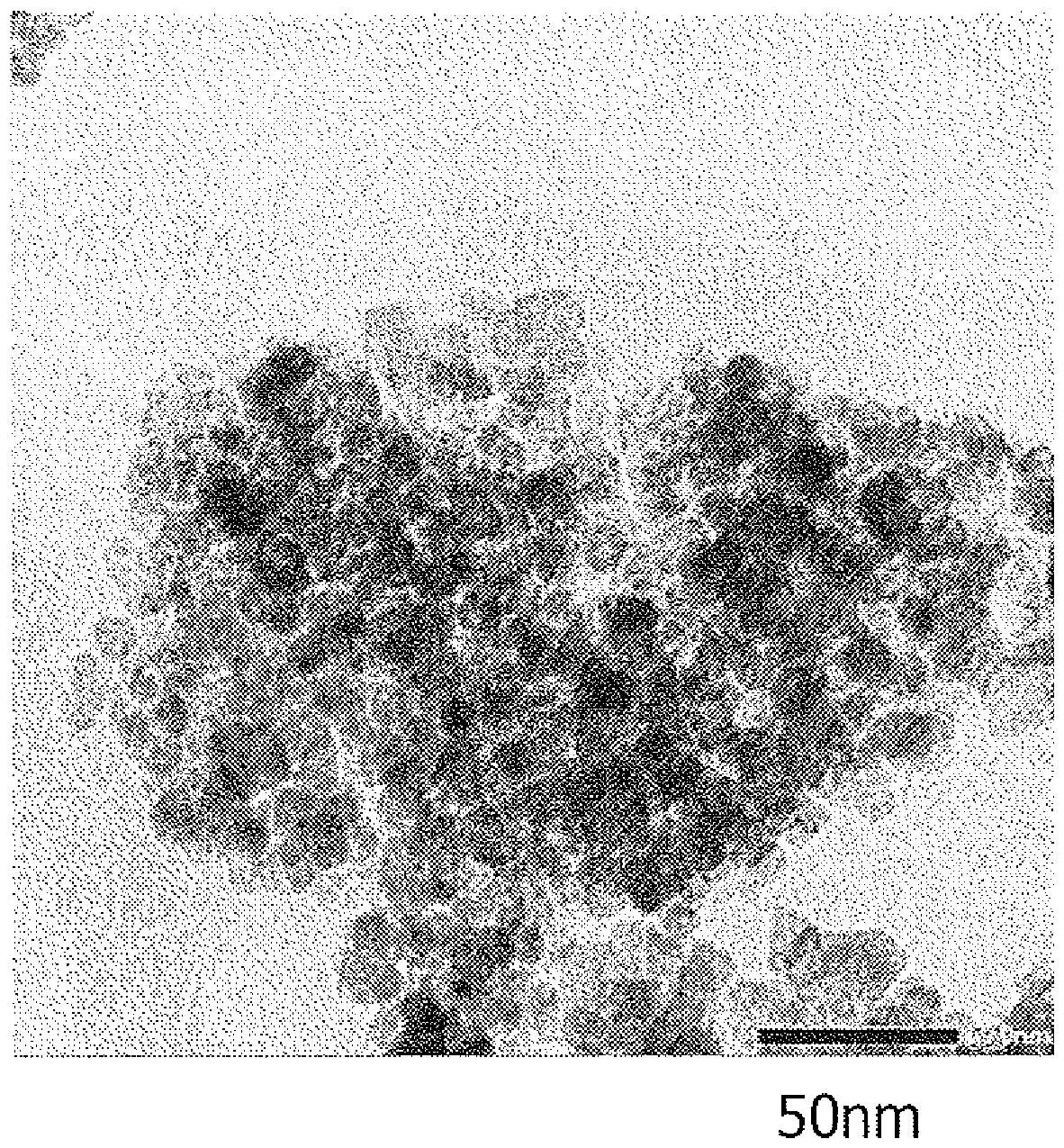
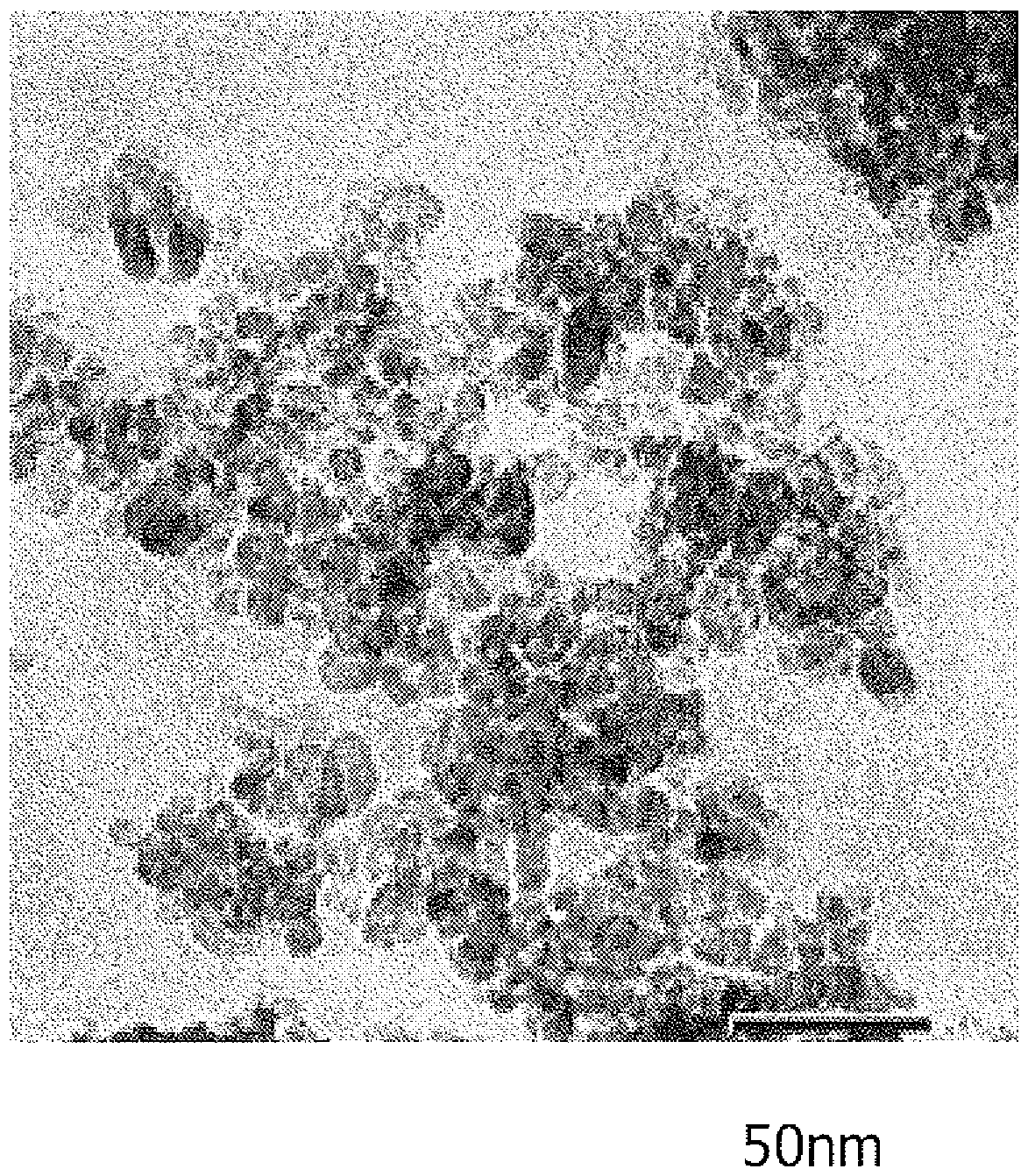
Method for producing titanium hydroxide
A technology of titanium hydroxide and manufacturing method, applied in chemical instruments and methods, titanium oxide/hydroxide, inorganic chemistry, etc., can solve the problem of high manufacturing cost of alkoxide method and achieve the effect of high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0067] Hereinafter, the present invention will be described in detail by listing Examples together with Reference Examples and Comparative Examples. It should be noted that the reference example is an example carried out in order to study the relationship between the conditions of simultaneous neutralization of the titanium tetrachloride aqueous solution and the alkaline substance in the step A and the BET specific surface area and crystallite diameter of the obtained titanium hydroxide. .
[0068] Generally speaking, the composition of titanium hydroxide obtained by neutralizing titanium halide in water with an alkaline substance, or the amount of water and water is uncertain. Therefore, the concentration of the titanium hydroxide in the water slurry is determined based on the weight of such titanium hydroxide, or the phosphorus compound and / or the phosphorus compound to be added to the titanium hydroxide is determined based on the weight of such titanium hydroxide. The prop...
reference example 1
[0071] (Process A)
[0072] will be TiO 2 Titanium tetrachloride aqueous solution with a concentration of 80 g / L and ammonia water with a concentration of 12.5 wt % were heated to 40°C, respectively. In another reaction vessel, 8 L of pure water heated to 40° C. was placed, and the above-mentioned titanium tetrachloride aqueous solution and ammonia water were simultaneously added thereto to carry out a neutralization reaction of titanium tetrachloride to precipitate titanium hydroxide to obtain an aqueous slurry. . The above-mentioned neutralization reaction was performed at a temperature of 50° C. for 4 hours in the range of pH 4.8 to 5.2. After that, the obtained water slurry was further stirred at a temperature of 40°C for 4 hours.
[0073] The water slurry thus obtained was cooled to room temperature, filtered and washed with water to obtain a titanium hydroxide cake. The obtained titanium hydroxide cake was dried at 120° C. for 15 hours to obtain titanium hydroxide po...
reference example 2
[0075] (Process A)
[0076] will be TiO 2 Titanium tetrachloride aqueous solution with a concentration of 80 g / L and ammonia water with a concentration of 12.5 wt % were heated to 40°C, respectively. In another reaction vessel, 8 L of pure water heated to 40° C. was placed, and the above-mentioned titanium tetrachloride aqueous solution and ammonia water were simultaneously added thereto to carry out a neutralization reaction of titanium tetrachloride to precipitate titanium hydroxide to obtain an aqueous slurry. . The said neutralization reaction was performed at the temperature of 40 degreeC in the range of pH 7.8-8.2 for 4 hours. After that, the obtained water slurry was further stirred at a temperature of 40°C for 4 hours.
[0077] The treated water slurry thus obtained was cooled to room temperature, filtered and washed with water to obtain a titanium hydroxide cake. The obtained titanium hydroxide cake was dried at 120° C. for 15 hours to obtain titanium hydroxide po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Crystallite diameter | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com