A method for efficiently producing chondroitin sulfate a by artificial enzymatic method
A technology of chondroitin sulfate and chondroitin, which is applied in the field of bioengineering, can solve the problems of small reaction scale, high cost, and long catalytic reaction time, and achieve the effects of quality and safety assurance, improved synthesis efficiency, and uniform product structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Construction of recombinant Pichia pastoris GS115 / lin1, GS115 / lin2, GS115 / lin3, GS115 / lin4
[0050] 1. Optimization of the gene encoding chondroitin 4-O-sulfotransferase
[0051] (1) First remove the N-terminal 1-62 amino acids from the sequence of GenBank accession number NP_067414.2, then perform codon optimization and artificial synthesis, and assemble and connect the synthesized original sequence to the pPIC9K plasmid to obtain the pPIC9K-Δ62C4ST plasmid. The plasmid was linearized with fast cutting enzyme SalI and then transformed into Pichia pastoris GS115 to obtain recombinant strain S001;
[0052] (2) The SUMO tag protein sequence was fused to the N-terminal of the Δ62C4ST sequence by fusion PCR technology, and then the fused sequence SUMOC4 was connected to the pPIC9K vector by Gibson assembly to obtain the pPIC9K-SUMOC4ST plasmid, which was linearized with the fast cutting enzyme SalI Transformed into Pichia pastoris GS115 after transformation to o...
Embodiment 2
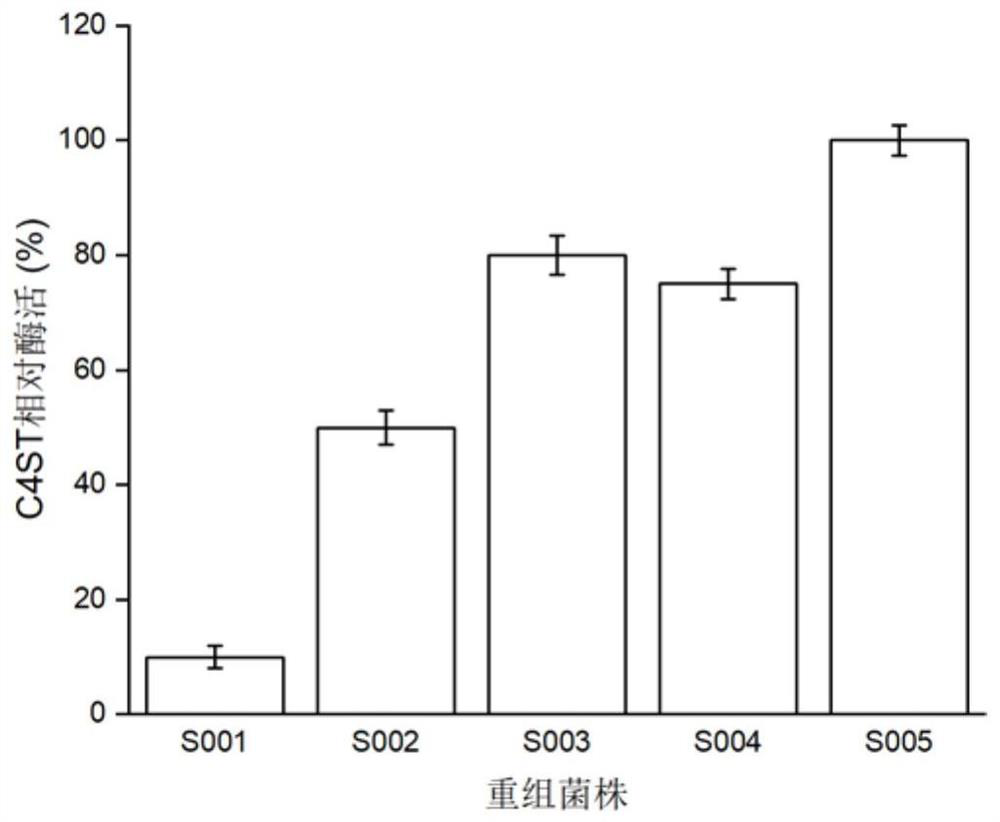
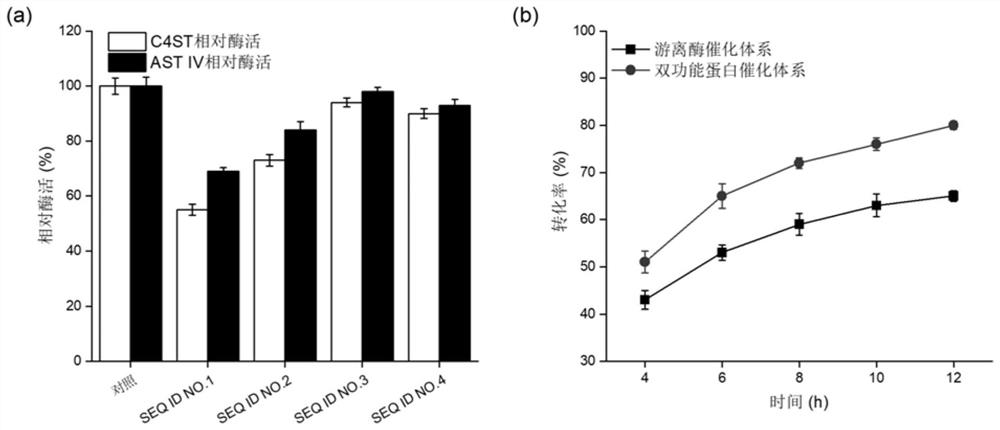
[0067] Embodiment 2: the comparison of two kinds of catalyst systems chondroitin sulfate A transformation rate
[0068] The engineering strains GS115 / lin1, GS115 / lin2, GS115 / lin3, and GS115 / lin4 constructed in Example 1 were respectively streaked on the YPD plate for activation, and then a single colony was inoculated in the YPD medium for 16 hours at 30°C, and then pressed for 10 % inoculum size Inoculated in BMGY medium, cultured at 30°C for 24h. After the cultivation, collect the bacteria, resuspend and wash the bacteria twice with physiological saline, and finally resuspend the bacteria in BMMY medium for cultivation at 30°C, add 1% methanol every 24 hours to induce expression, and induce the culture for 96 hours Afterwards, the supernatant was collected by centrifugation, and the enzyme activities of C4ST and AST IV were determined. Using freely expressed C4ST and AST IV as controls, the relative enzyme activity was calculated, and the results were as follows: figure 2...
Embodiment 3
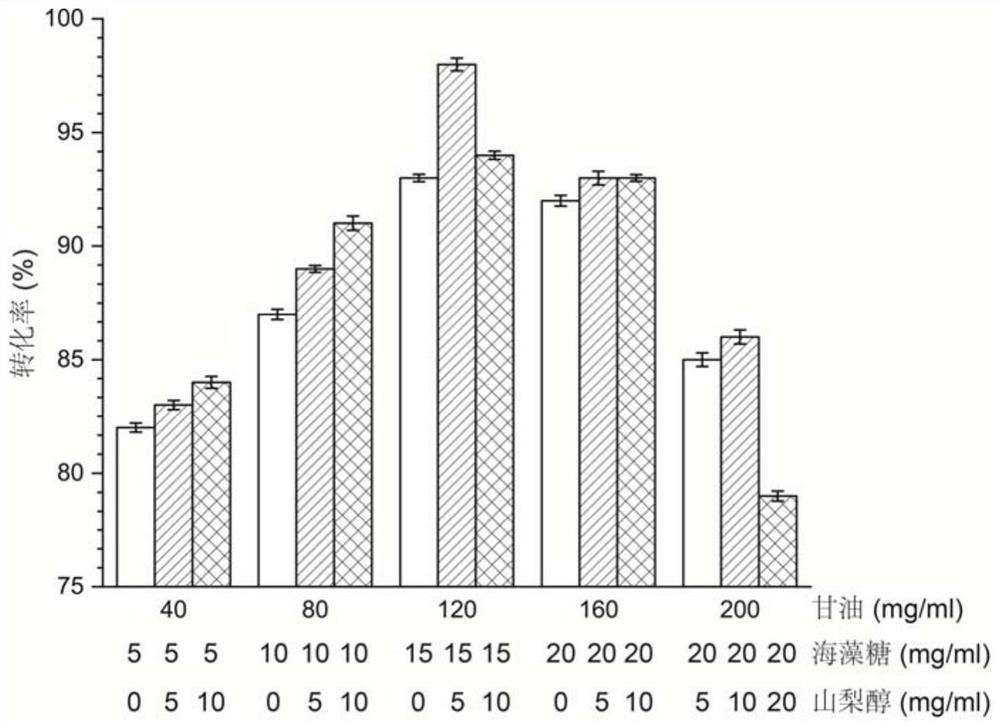
[0073] Example 3: Steps for obtaining the bifunctional protein lin3
[0074] Inoculate a single colony of the recombinant strain GS115 / lin3 in 5mL YPD liquid medium, culture at 30°C for 24h, then transfer 10% of the inoculum to a shaker flask containing 50mL of BMMY medium, and culture at 30°C for 24h The bacteria were collected by centrifugation, washed twice with sterile saline, resuspended in 50 mL of BMGY medium, and cultured at 25°C for 96 hours, during which 1% volume of methanol was added every 24 hours to induce protein expression.
[0075] After shaking the flask, the supernatant of the fermentation broth was collected by centrifugation, which was the crude enzyme solution of the bifunctional protein lin3. The bifunctional protein lin3 was eluted and purified through a nickel column under the conditions of phosphate buffer (pH 7.0) containing 300mM imidazole and 500mM NaCl. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com