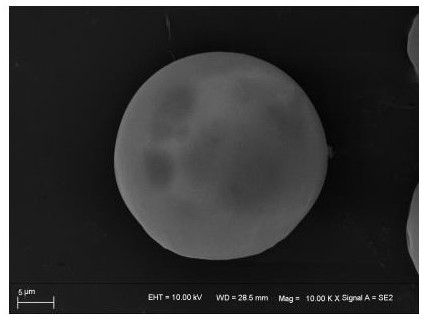
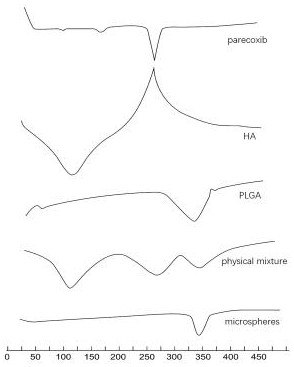
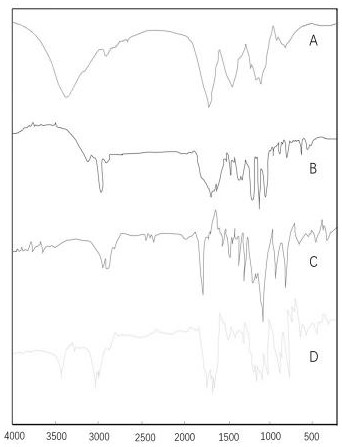
Hyaluronic acid-parecoxib PLGA microsphere as well as preparation method and application thereof
A technology of hyaluronic acid and parecoxib, which is applied in the field of preparation of new sustained-release microspheres, which can solve the problems of repeated injections and achieve the effect of uniform size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] A preparation method of hyaluronic acid-parecoxib PLGA microspheres, comprising the following steps:
[0037] a. Modify the hydrophilic hyaluronic acid, and transform it into an amphiphilic polymer by grafting hyaluronic acid with stearic acid, which is used as a surfactant in the subsequent microsphere preparation process;
[0038] b. Using double emulsification solvent volatilization method, dissolve PLGA in an appropriate amount of organic solvent, add a small amount of aqueous solution containing water-soluble drugs in proportion, add modified hyaluronic acid, and process it by ultrasonic or vibration to obtain the state Stable W / O emulsion;
[0039] c. Add a large amount of aqueous phase solution for the second emulsification treatment to obtain a W / O / W emulsion;
[0040] d. Remove the organic solvent by stirring and evaporating to obtain a microsphere suspension, and freeze-dry to obtain a microsphere powder.
[0041] Specifically, a preparation method of hyalur...
Embodiment 1
[0047] A preparation method of hyaluronic acid-parecoxib PLGA microspheres, comprising the following steps:
[0048] 1) Dissolve 200 mg of hyaluronic acid in 5 ml of deionized water, 80 mg of stearic acid in 25 ml of DMSO, add EDC to activate, mix the two solutions, add DMAP and stir overnight, precipitate with 300 ml of ethanol, and freeze-dry.
[0049] 2) Dissolve 40 mg parecoxib in 0.2 ml deionized water to form a uniform inner water phase, dissolve 240 mg PLGA in 2 ml dichloromethane as an oil phase, add the oil phase to the inner water phase, and add Amphiphilic hyaluronic acid (as surfactant).
[0050] 3) Immerse in an ice-water bath and stir for 1 min at high speed with a ultrasonic emulsifier to obtain colostrum. Drop the colostrum into 8ml of 4% PVA solution at a uniform speed and stir at high speed for 2 min to obtain double milk.
[0051]4) Transfer the double emulsion to a large volume of 100 ml 4% PVA aqueous solution, stir at a low speed for 3 hours to completel...
Embodiment 2
[0053] Example 2 Determination of drug loading and encapsulation efficiency
[0054] Weigh the weight of drug-loaded microspheres after lyophilization. Accurately weigh 20mg of microspheres and place them in a centrifuge tube, add 2ml of dichloromethane, and ultrasonically shake for 2 minutes to dissolve the microspheres; then dilute to 10ml with water, shake for 1 minute, let stand for 5 minutes, centrifuge at 10,000 rpm for 10 minutes, and take the supernatant ; Repeat the above steps twice more, collect all the supernatant, and measure the absorbance value (A) with a UV spectrophotometer, drug loading = (mass of drug in microspheres / mass of drug-containing microspheres) × 100%, Encapsulation efficiency = (actual drug content in microspheres / theoretical drug content in microspheres) × 100%.
[0055] The drug loading rate of the microsphere obtained by the present invention is 20.95%, and the encapsulation rate is 54.70%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com