Application of CQMU151 in preparation of medicine for treating Th17 cell mediated autoimmune diseases
An autoimmune disease, cell technology, applied in the field of medicine, can solve problems such as carcinogenesis, toxic side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
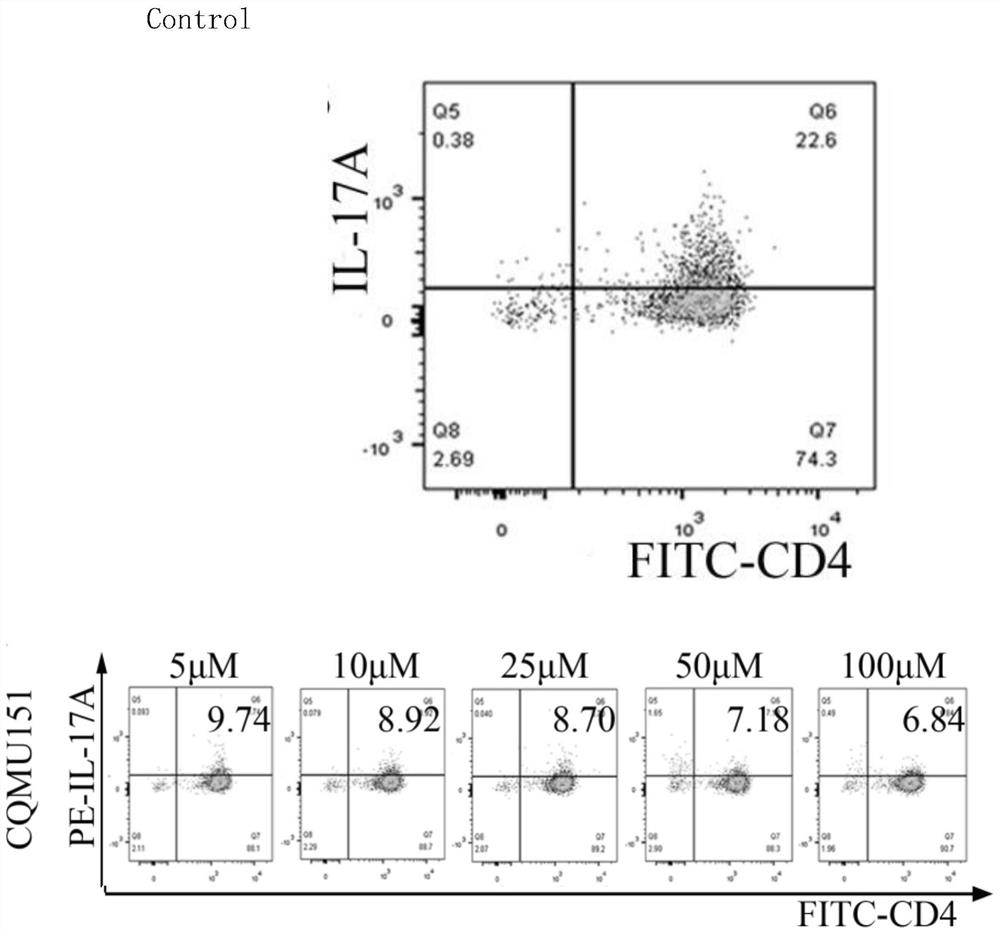
[0023] Example 1 N-((1H-benzo[d]imidazol-2-yl)methyl)-3-(morpholinesulfonyl)benzamide inhibits initial CD4 in vitro + Differentiation of T cells into Th17 cells
[0024] 1. Experimental method:
[0025] 1) After the mice were sacrificed, they were soaked in 75% ethanol, and the spleen was dissected and separated into a filter in an ultra-clean bench.
[0026] 2) Rinse and filter with PBS after grinding with the rubber tip of a 5ml syringe. Collect the filtrate into a 15ml centrifuge tube.
[0027] 3) Centrifuge at 4°C for 10 minutes with a centrifugal force of 350*g, discard the supernatant, add red blood cell lysate at a volume 5 times the volume of the cell, blow it gently, let it stand for 2 minutes, and then set it at 4°C with a centrifugal force of 350*g Centrifuge for 5 minutes and discard the supernatant.
[0028] 4) Add the same amount of cold PBS as the erythrocyte lysate, after blowing gently, centrifuge at 4°C for 2 minutes at a centrifugal force of 350*g, disca...
Embodiment 2
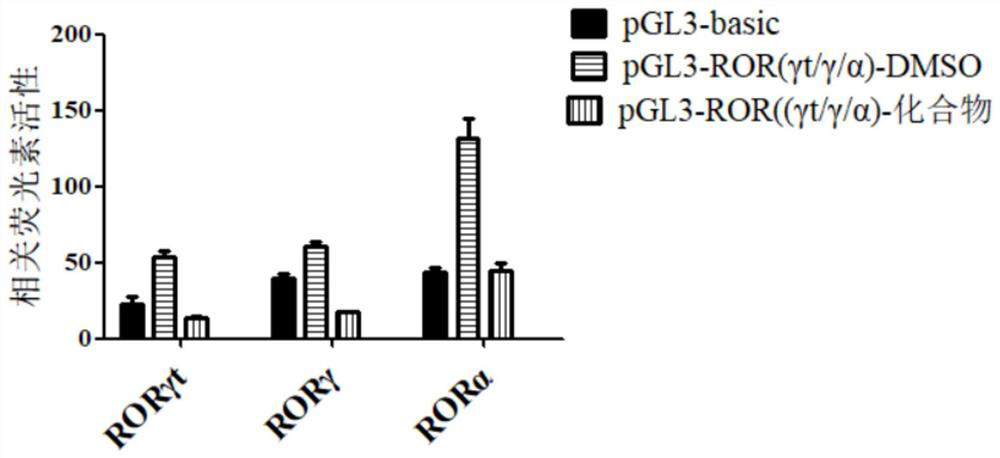
[0031] Example 2 The binding test results of N-((1H-benzo[d]imidazol-2-yl)methyl)-3-(morpholinesulfonyl)benzamide and target RORγt
[0032] 1. Experimental method:
[0033] Using the double luciferase method (Yang X O, et al.T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and RORgamma.Immunity 28,29-39(2008)), construct RORγt overexpression plasmid and IL-17 promoter daughter reporter plasmid. Divided into three groups: the blank group without reporter gene, the control group of reporter gene + RORγt overexpression plasmid + DMSO, and the experimental group of reporter gene + RORγt overexpression plasmid + compound interference, and the plasmid was transduced into 293T engineered cells. After 16 hours, the medium was changed and DMSO / compounds were added for interference. Express 2 days.
[0034] After 48 hours, the cells were lysed according to the procedure of Promega's dual-luciferase detection kit, and the fluorescence values of...
Embodiment 3
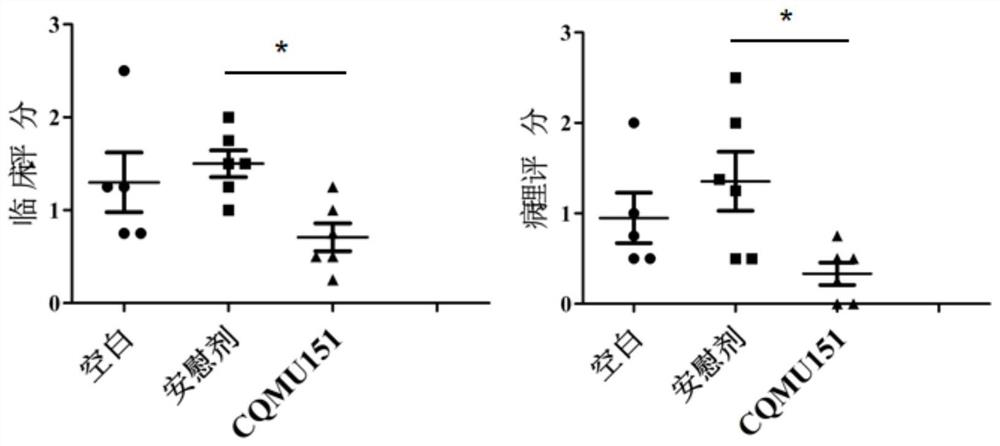
[0036] Example 3 Effect of N-((1H-benzo[d]imidazol-2-yl)methyl)-3-(morpholinesulfonyl)benzamide on disease severity of EAU in mice in vivo
[0037] 1. Experimental method:
[0038] Antigen preparation: weigh 5 mg of IRBP with a microbalance 651-670 Put the powder in an EP tube, add 1ml of sterile PBS containing 2% DMSO to the EP tube containing IRBP with a pipette, vibrate on a shaker to accelerate dissolution, and then centrifuge at 3000r / min for 30s on a speed centrifuge To discharge the air bubbles in the tube, prepare an IRBP solution with a concentration of 5 mg / ml, and store it at 4°C for later use.
[0039] Anesthetize 20 mice with sodium pentobarbital, connect 100ul (ie 500ng) IRBP solution (5mg / ml) with 100ul complete Freund's adjuvant containing 5mg / ml Mycobacterium tuberculosis through a sterile three-way tube and fully After mixing into a white emulsion, use the above mixed solution with a total volume of 200ul to inject subcutaneously at the back of the neck, bi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com