A kind of preparation method of roquinex
A technology of oxoquinoline and dihydrogen, which is applied in the preparation field of preparing a roquimec, can solve the problem of stability and mechanical strength, which have not been reported further, and that methanol cannot be completely separated from the system, affecting product content and yield. and other problems, to achieve the effect of shortening the reaction time, reducing the amount of solvent used, and improving the yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
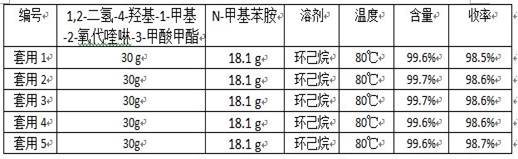
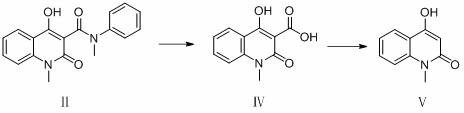
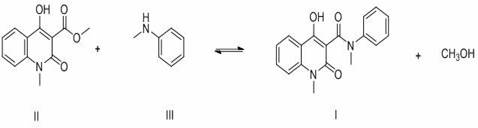
[0032] 30.0 g 1,2-dihydro-4-hydroxy-1-methyl-2-oxoquinoline-3-carboxylic acid methyl ester (0.13 mol), 18.1 g N-methylaniline (0.17 mol) and 120 g Add n-heptane and 1.2 g of anhydrous calcium chloride into a 250 mL three-necked flask, connect the flask to the inlet of a type A molecular sieve pervaporation inorganic membrane device, stir and heat to reflux, and reflux for 3.5 h. During the reaction, the methanol in the organic vapor and The water mixture passes through the A-type molecular sieve to infiltrate and vaporize the inorganic membrane tube to separate the system, and the solvent is refluxed back to the kettle for reaction. At the end of the reaction, a total of 5.1 g of methanol and water were collected. The reaction solution was filtered at 70°C, the filter cake was used as a catalyst, the filtrate was cooled and crystallized and filtered, and the filter cake was dried to obtain 39.2 g of white solid with a content of 99.6% (external standard of liquid chromatograph...
Embodiment 2
[0034] 30.0 g 1,2-dihydro-4-hydroxy-1-methyl-2-oxoquinoline-3-carboxylic acid methyl ester (0.13 mol), 18.1 g N-methylaniline (0.17 mol) and 180 g Cyclohexane and 1.8 g of anhydrous calcium chloride were added to a 250 mL three-necked flask, and the flask was connected to the inlet of a type A molecular sieve pervaporation inorganic membrane device, stirred and heated to reflux, and refluxed for 2.5 h. During the reaction, the methanol in the organic vapor and The water mixture passes through the A-type molecular sieve to infiltrate and vaporize the inorganic membrane tube to separate the system, and the solvent is refluxed back to the kettle for reaction. At the end of the reaction, a total of 5.1 g of methanol and water were collected. The reaction solution was filtered at 60°C, the filter cake was used as a catalyst (for the next batch of reactions), the filtrate was cooled, crystallized and filtered, and the filter cake was dried to obtain 39.3 g of white solid, with a con...
Embodiment 3
[0036] 30.0 g 1,2-dihydro-4-hydroxy-1-methyl-2-oxoquinoline-3-carboxylic acid methyl ester (0.13 mol), 14.9 g N-methylaniline (0.14 mol) and 150 g Add n-heptane and 1.5 g of anhydrous calcium chloride into a 250 mL three-neck flask, connect the flask to the inlet of a type A molecular sieve pervaporation inorganic membrane device, stir and heat to reflux, and reflux for 3 h. During the reaction, methanol and The water mixture passes through the A-type molecular sieve to infiltrate and vaporize the inorganic membrane tube to separate the system, and the solvent is refluxed back to the kettle for reaction. After the reaction, a total of 5.2 g of methanol and water were collected. The reaction solution was filtered at 65°C, the filter cake was used as a catalyst, the filtrate was cooled and crystallized and filtered, and the filter cake was dried to obtain 39.3 g of white solid with a content of 99.5% (LC external standard) and a yield of 98.5% (with 1,2-dihydro -4-Hydroxy-1-met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com