Preparation method of mecobalamin dispersible tablets
A technology of methylcobalamin and dispersible tablets, applied in the field of medicinal chemistry, can solve problems such as instability, and achieve the effects of high production efficiency, high purity and risk reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
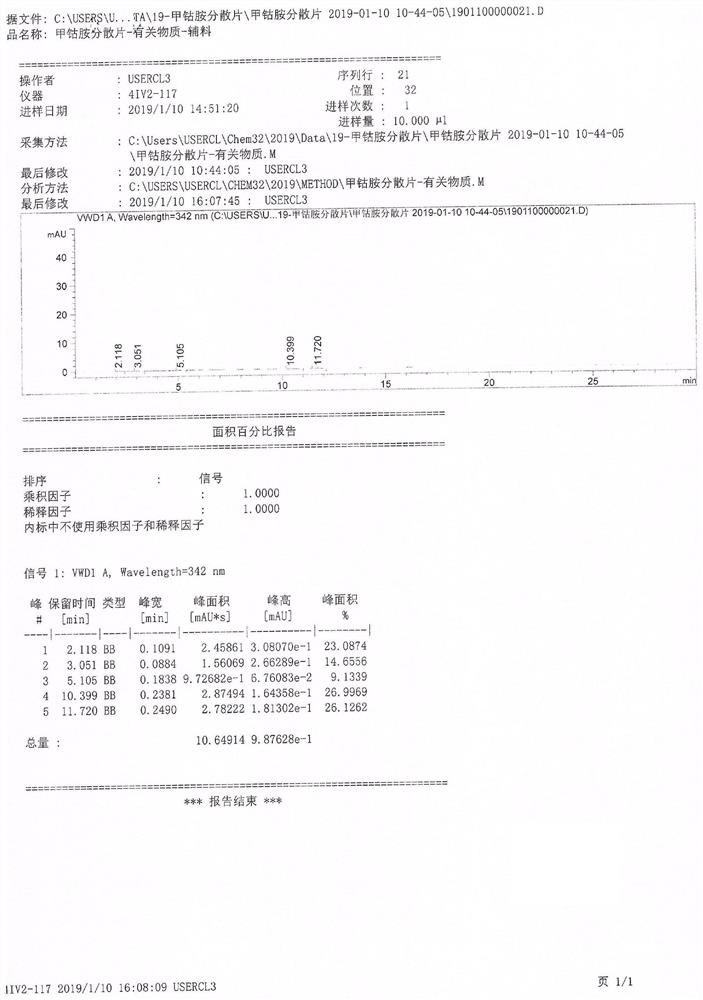
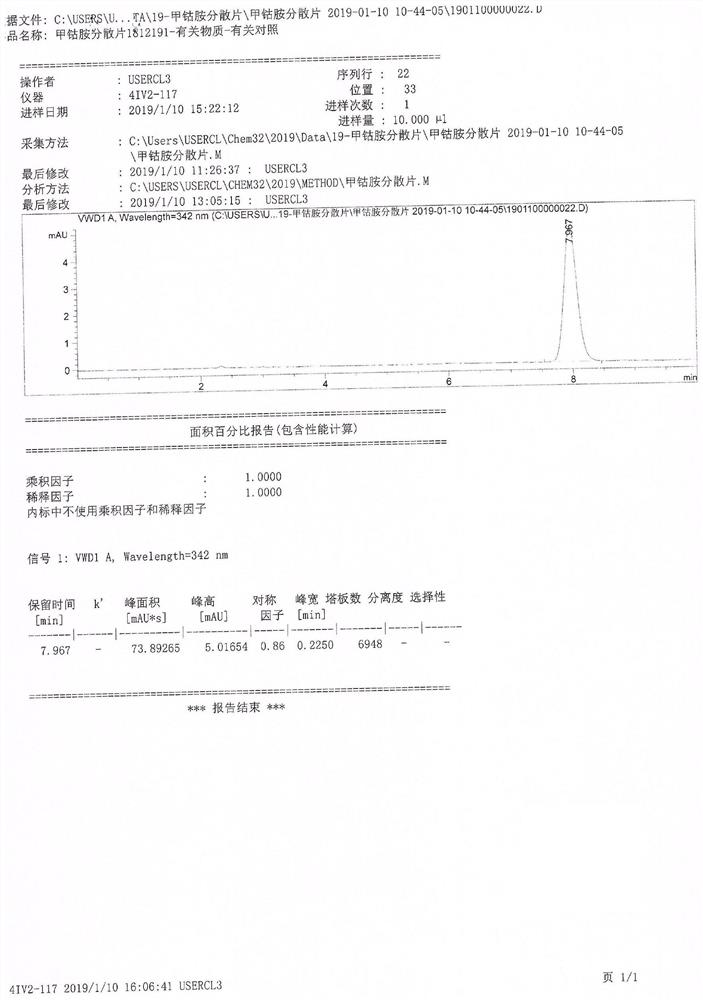
Image
Examples
Embodiment 1
[0031] This embodiment discloses a preparation method of methylcobalamin dispersible tablets, comprising the following steps:
[0032] Step 1: Prepare the following materials for later use: methylcobalamin raw material, microcrystalline cellulose, lactose, croscarmellose sodium, and magnesium stearate. Among them, 0.5g of methylcobalamin raw material, 88.9g of microcrystalline cellulose, 28.8g of lactose, 1.2g of croscarmellose sodium, and 0.6g of magnesium stearate were weighed under the condition that the ambient humidity was 40%. the above materials.
[0033] Step 2: pass the raw material of methylcobalamin through a standard sieve, the standard sieve is a 60-mesh sieve; divide the microcrystalline cellulose into two parts, the first part of microcrystalline cellulose is 13.3g, and the second part of microcrystalline cellulose is 75.6g.
[0034] Step 3: Add the first part of microcrystalline cellulose into the three-dimensional mixer under the condition of avoiding light t...
Embodiment 2
[0039] This embodiment discloses a preparation method of methylcobalamin dispersible tablets, comprising the following steps:
[0040] Step 1: Prepare the following materials for later use: methylcobalamin raw material, microcrystalline cellulose, lactose, croscarmellose sodium, and magnesium stearate. Among them, 0.5g of methylcobalamin raw material, 88g of microcrystalline cellulose, 28g of lactose, 2.5g of croscarmellose sodium, and 1g of magnesium stearate were weighed under the condition that the ambient humidity was 35%.
[0041] Step 2: pass the methylcobalamin raw material through a standard sieve, which is a 40-mesh sieve; divide the microcrystalline cellulose into two parts, the first part of microcrystalline cellulose is 15g, and the second part of microcrystalline cellulose is 73g.
[0042] Step 3: Add the first part of microcrystalline cellulose into the three-dimensional mixer under the condition of avoiding light throughout the process, then add the methylcobala...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com