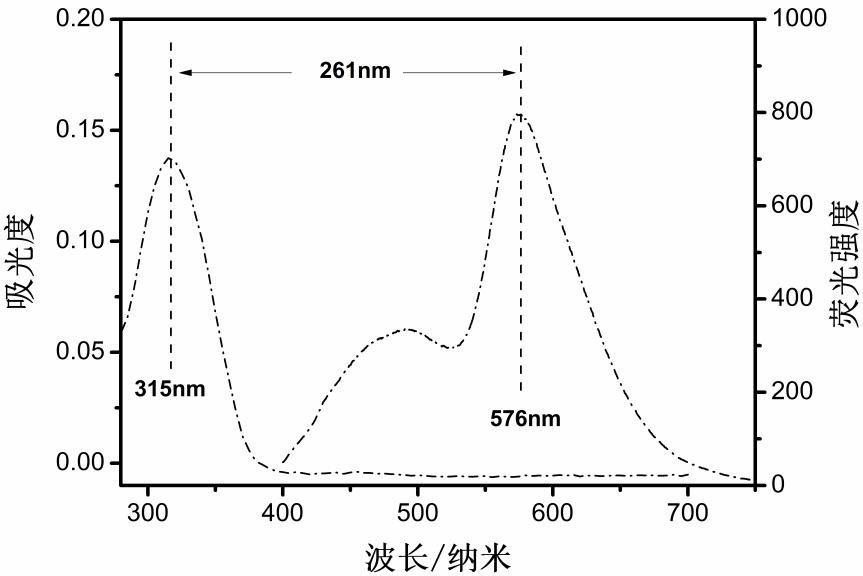
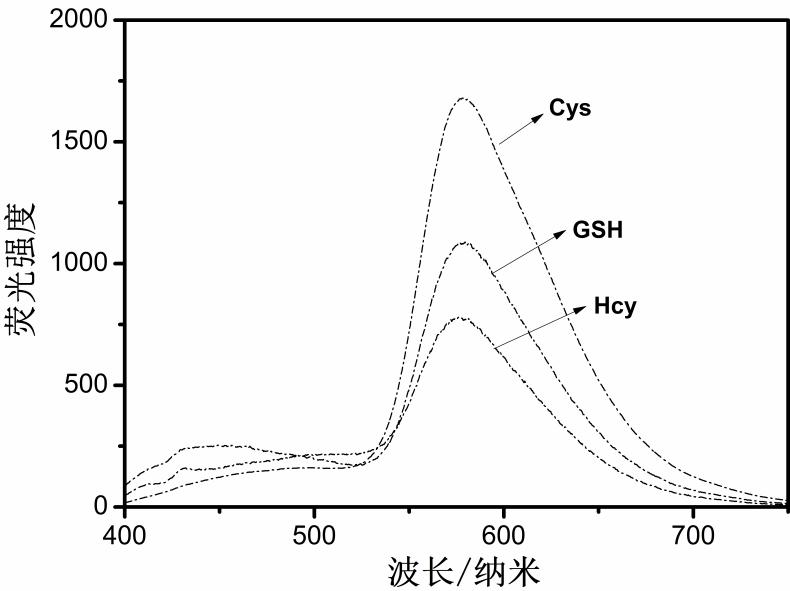
Biflavone derivative fluorescent probe, preparation method thereof and application of biflavone derivative fluorescent probe in brain glioma imaging
A technology of fluorescent probes and derivatives, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problem of lack of fluorescent probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
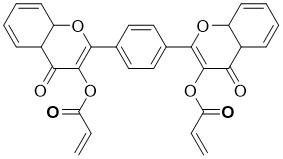
[0036] Step 1. Compound (II): Preparation of 2,2'-(1,4-phenylene)bis(3-hydroxy-4a,8a-dihydro-4H-chromium-4-one)
[0037] 2-Hydroxyacetophenone (0.68g, 5 mmol) and terephthalaldehyde (0.67g, 5 mmol) were dissolved in 40 ml of methanol and NaOH (1.5g, 0.0375mmol) was added, heated to reflux for 6 h, cooled to The mixture was stirred at room temperature for 12h. Then NaOH solution (0.5 mol / L, 5 mL) and 30% H 2 o 2 Solution (9.9mol / L, 5 mL), the reaction solution was stirred at room temperature for 6 h, poured into ice water (200 mL) after the reaction was completed, put it in the refrigerator for 6 h, and filtered to obtain intermediate compound (Ⅱ) 2 ,2'-(1,4-phenylene)bis(3-hydroxy-4a,8a-dihydro-4H-chromium-4-one). 1 H NMR (400 MHz, DMSO-d6) δ 8.71(s, 4H), 8.05 (d, J = 8.0 Hz, 2H), 7.58 (dd, J = 20.2, 7.9 Hz, 4H), 7.24 (t, J = 7.6 Hz, 2H).
[0038]
[0039] Step 2. Preparation of probe molecule 1,4-phenylbis(4-oxo-4H-benzopyran-2,3-diyl)diacrylate (PPDC)
[0040] Weigh t...
Embodiment 2
[0043] The first step, the preparation of compound (II): 2,2'-(1,4-phenylene)bis(3-hydroxy-4a,8a-dihydro-4H-chromium-4-one) is the same as in Example 1 same.
[0044] Step 2. Preparation of probe molecule 1,4-phenylbis(4-oxo-4H-benzopyran-2,3-diyl)diacrylate (PPDC)
[0045] Weigh the intermediate compound (Ⅱ) 2,2'-(1,4-phenylene)bis(3-hydroxy-4a,8a-dihydro-4H-chromium-4-one) (0.3986 g, 1 mmol), Add 40 ml of n-hexane, add hexahydropyridine (300 µL, 2 mmol) under ice-water bath, react for 40 min, then add acryloyl chloride (180 µL, 2 mmol) dropwise, and continue to react for about 30 min under ice-water bath , and then raised to room temperature to react, and the end point of the reaction was monitored by thin-layer chromatography (TLC) spotting. After about 10 h, the reaction was completed (the reaction solution changed from red to orange). After the reaction was completed, the filtrate was spin-dried by a rotary evaporator, and eluted with ethyl acetate:petroleum ether=1:3 t...
Embodiment 3
[0047] The first step, the preparation of compound (II): 2,2'-(1,4-phenylene)bis(3-hydroxy-4a,8a-dihydro-4H-chromium-4-one) is the same as in Example 1 same.
[0048] Step 2. Preparation of probe molecule 1,4-phenylbis(4-oxo-4H-benzopyran-2,3-diyl)diacrylate (PPDC)
[0049] Weigh the intermediate compound (Ⅱ) 2,2'-(1,4-phenylene)bis(3-hydroxy-4a,8a-dihydro-4H-chromium-4-one) (0.3986 g, 1 mmol), Add 40 ml of ethanol, add sodium ethoxide (450 µL, 3 mmol) under ice-water bath, and react for 40 min, then add acryloyl chloride (240 µL, 3 mmol) dropwise, continue to react for about 30 min under ice-water bath, and then rise to React at room temperature, and monitor the end point of the reaction by spotting a thin-layer chromatography (TLC) plate. After about 10 h, the reaction ends (the reaction solution changes from red to orange). After the reaction was completed, the filtrate was spin-dried by a rotary evaporator, and eluted with ethyl acetate:petroleum ether=1:3 to obtain the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com