Beta-galactosidase near-infrared fluorescent probe, preparation method and application thereof
A galactosidase and fluorescent probe technology, applied in the field of biological detection, achieves the effect of stable photophysical activity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
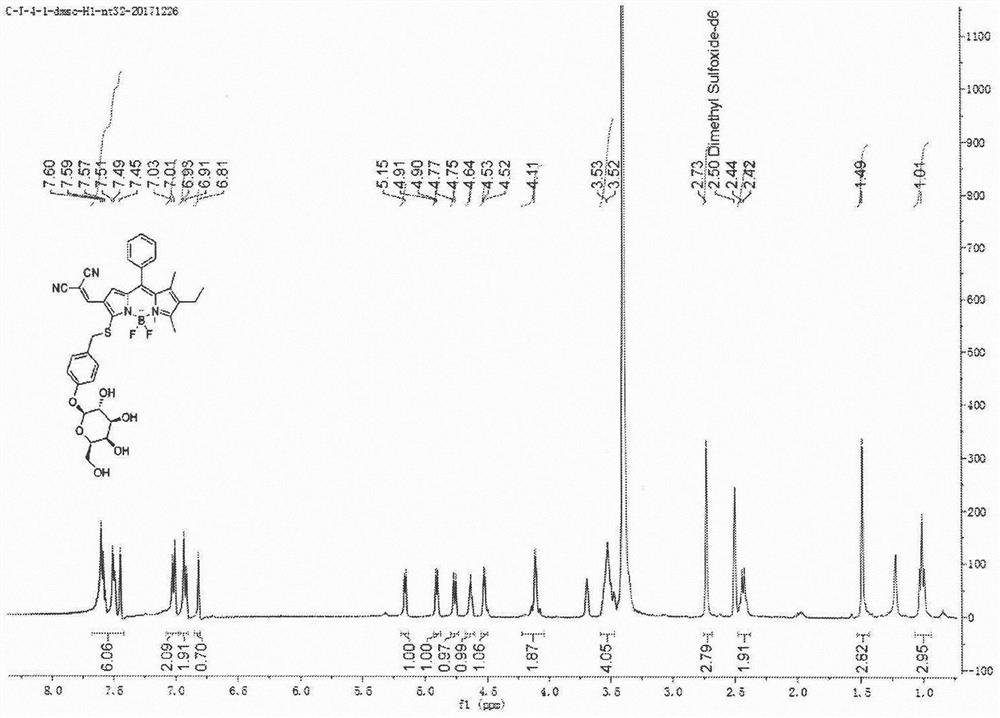
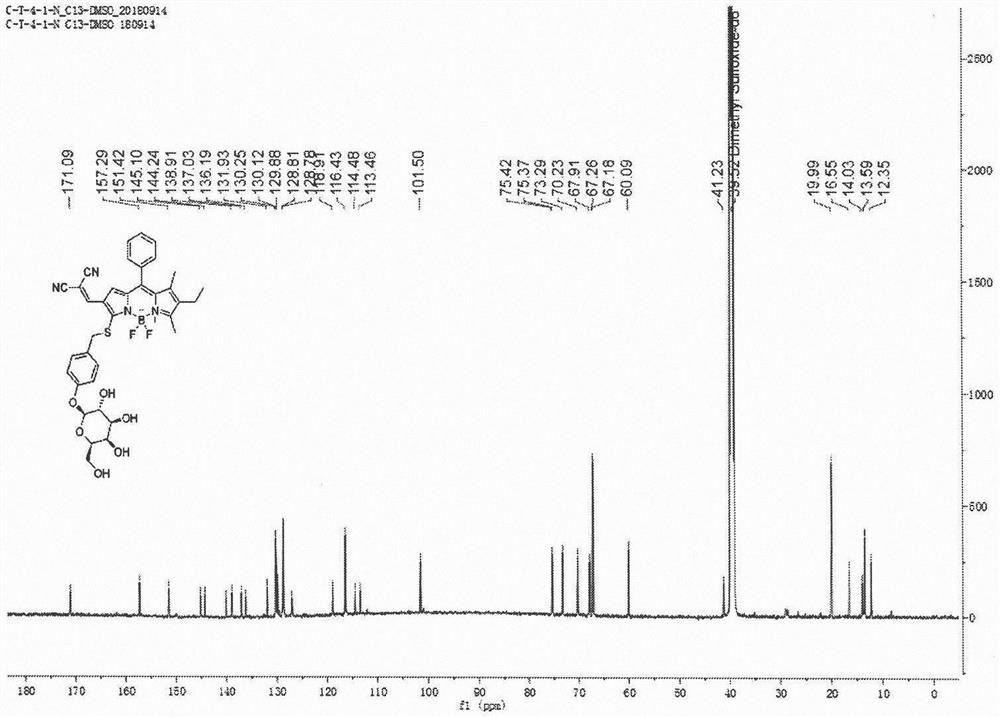
[0070] Synthetic probe general formula (1), its structure is:
[0071]
[0072] The synthesis process of the probe is as follows:
[0073]
[0074] 1) Preparation of Compound C:
[0075]
[0076] Boron trifluoride ether solution (1.8 mL, 13.2 mmol) was added to compound B (12 g, 96 mmol) in thioglycolic acid (30 mL), reacted at room temperature for 3 h, and monitored by TLC until the reaction was complete. The solution was diluted with ethyl acetate, washed with saturated brine, and the organic phase was subjected to silica gel column chromatography to obtain Compound C (14 g, 80%) as a yellow oil. 1 H NMR (400MHz, CDCl 3 ), δ2.27(s, 3H), 3.99(s, 2H), 5.66(s, 1H), 6.98(d, J=8.1Hz, 2H), 7.07(d, J=8.1Hz, 2H).ESI -MS calculated for C 9 h 10 NaO 2 S + [M+Na] + : 205.03, found: 205.1.
[0077] 2) Preparation of compound D:
[0078]
[0079] The mixture of compound C (11.7g, 64mmol) and A-D-pentaacetylgalactose (12.5g, 32mmol) was dissolved in DMF (100ml), and ...
Embodiment 2
[0095] Example 2 Changes in UV absorption spectra before and after the reaction between probe C-1 and β-galactosidase
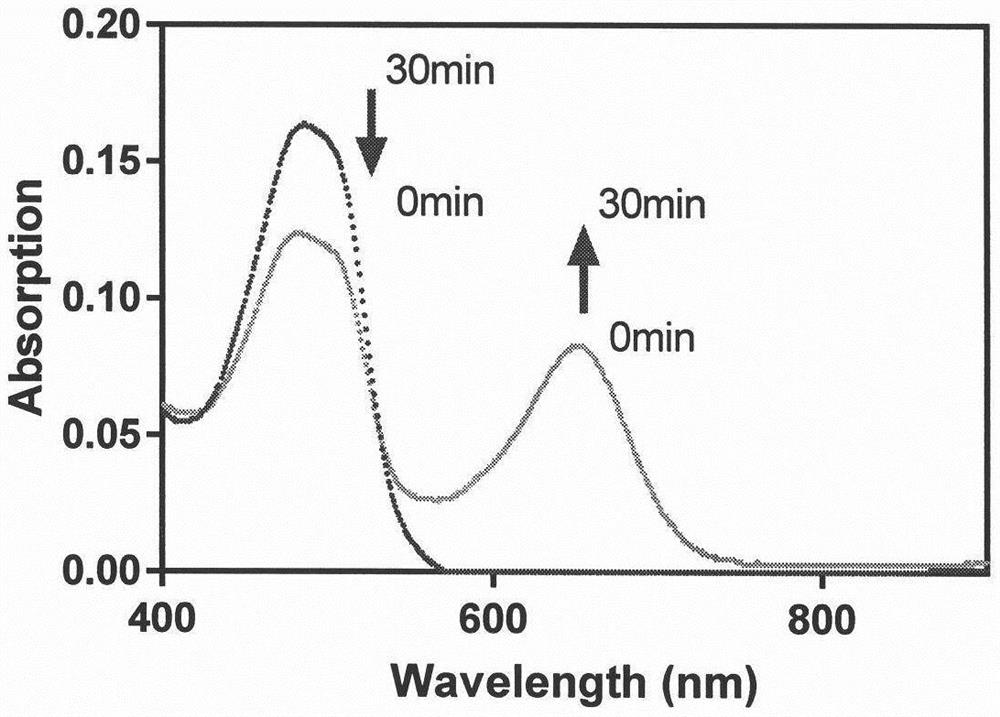
[0096] Prepare 30% DMSO in PBS solution for immediate use; prepare the probe into 1mM acetonitrile solution for later use; prepare β-galactosidase (1mg, 1580u / mg) with deionized water into 1000u / mL stock solution, aliquoted and stored at -20°C, for later use, add 3mL of 30% DMSO PBS (pH 7.4, 50mM) buffer solution to a 3mL quartz cuvette, then add 10μL of probe, 14μL of β-galactoside Enzyme, react 30min under 37 ℃, measure its absorption spectrum, by image 3 It can be seen that after the reaction of probe C-1, the maximum ultraviolet absorption intensity at 488 nm becomes weaker, and the maximum ultraviolet absorption intensity at 651 nm becomes stronger.
Embodiment 3
[0097] Example 3. Fluorescence emission spectrum changes before and after the reaction between probe C-1 and β-galactosidase
[0098] Add 3mL of 30% DMSO PBS (pH 7.4, 50mM) buffer solution to a 3mL quartz cuvette, then add 10μL of probe and 14μL of β-galactosidase in turn, react at 37°C for 30min, and measure its absorption Spectrum, react at 37°C for 300min, measure its fluorescence emission spectrum, excitation wavelength 488m, excitation slit width 5nm, emission slit width 5nm, gain 700V; excitation wavelength 651nm, excitation slit width 10nm, emission slit width 10nm, gain 700V, Figure 4 It shows that after adding β-galactosidase, the fluorescence at 574nm decreases to 60% of the original, and the fluorescence at 727nm increases to 34.8 times of the original.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com