Nonaqueous electrolyte solution and energy device using same
A technology of non-aqueous electrolyte and energy equipment, which is applied in the direction of non-aqueous electrolyte batteries, non-aqueous electrolytes, electrolytic capacitors, etc., and can solve the problems of high battery capacity, increased battery voltage, insufficient battery impedance characteristics, and insufficient durability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0558] Hereinafter, the present invention will be described more specifically with reference to examples and comparative examples, but the present invention is not limited to these examples.
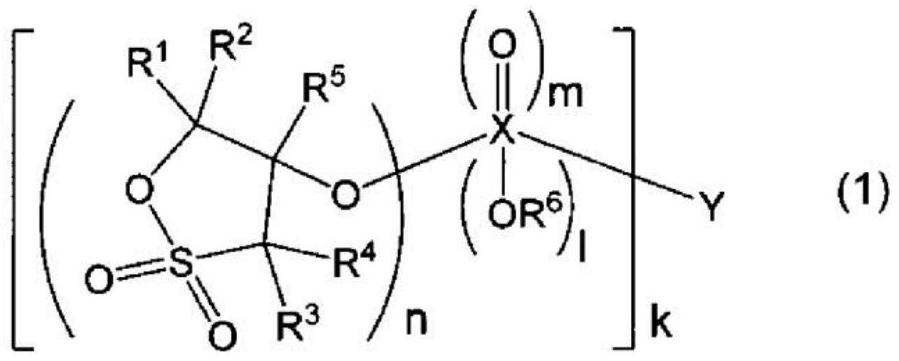
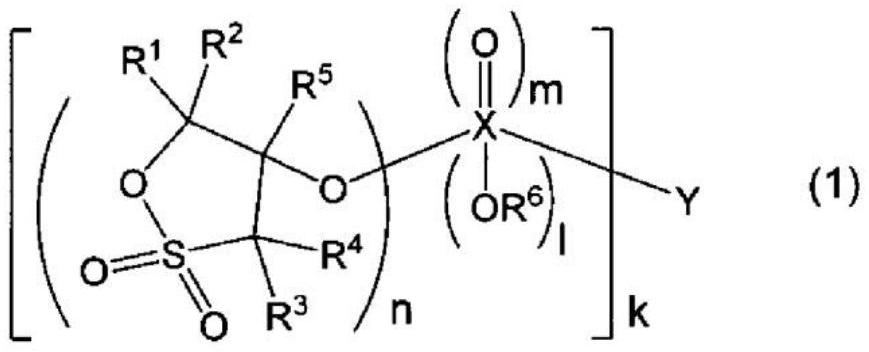
[0559] The structure of the compound represented by the formula (1) used in this example is shown below.
[0560] [chemical formula 33]
[0561]
[0562] Each substituent of formula (1): R 1 ~R 5 =H, X=S, Y=CH 2 , l=0, m=2, n=1, k=2.
[0563] [chemical formula 34]
[0564]
[0565] Each substituent of formula (1): R 1 ~R 5 =H, X=S, Y=C 4 h 8 , l=0, m=2, n=1, k=2.
[0566] [chemical formula 35]
[0567]
[0568] Each substituent of formula (1): R 1 ~R 5 =H, X=C, Y=none, l=0, m=1, n=1, k=2.
[0569] [chemical formula 36]
[0570]
[0571] (compound (1-4))
[0572] Each substituent of formula (1): R 1 ~R 5 =H, X=C, Y=CH 2 , l=0, m=1, n=1, k=2.
[0573] [chemical formula 37]
[0574]
[0575] Each substituent of formula (1): R 1 ~R 5 =H, X=C, Y=C 2 h 4 ...
Synthetic example 1
[0591]
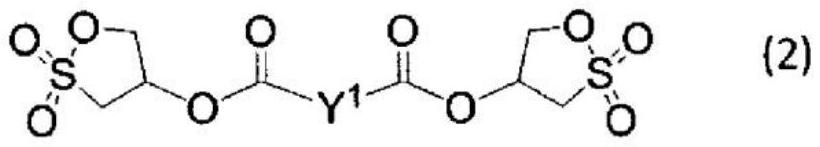
[0592] In a nitrogen atmosphere, 1.0 g (7.2 mmol) of 2-hydroxy-1,3-propane sultone was fed into a 50 ml three-necked flask, and dissolved in 20 ml of THF. It was cooled to <10° C. using an ice bath, and methanedisulfonyl dichloride 0.76 g (3.5 mmol) / THF 5 ml was added. Next, a solution of 0.81 g (8.0 mmol) of triethylamine / THF 10 ml was slowly added dropwise so that the internal temperature would not exceed 10°C. After stirring at <5°C for 2 hours, 20 ml of water was added, and extracted three times with 20 ml of dichloromethane. The obtained organic layer was concentrated until a white solid was precipitated, and the white solid was collected by filtration under reduced pressure and dried in vacuo. Compound (1-1) 0.38 g was obtained as a white solid, yield: 25.9%.
[0593] 1 H-NMR (DMSO-d6, 400MHz): 6.37(s,2H), 5.94-5.92(m,2H), 4.81-4.77(m,2H), 4.73-4.70(m,2H), 4.06-4.01(m ,2H),3.93-3.89(m,2H).MS(ESI):m / z 414.9(M-H) -
Synthetic example 2
[0595]
[0596] In a nitrogen atmosphere, 2.17 g (15.7 mmol) of 2-hydroxy-1,3-propane sultone was fed into a 100 ml three-necked flask, and dissolved in 30 ml of THF. It was cooled to <10°C using an ice bath and 2.4ml (17.2mmol) of triethylamine was added. A 2.0 g (7.8 mmol) / THF 20 ml solution of 1,4-butanedisulfonyl dichloride was slowly added dropwise thereto, while controlling so that the internal temperature did not exceed 10°C. After the dropwise addition, stirring was carried out at <10° C. for 2 hours. 30 ml of ethyl acetate / 20 ml of water were added thereto and stirred, and the insoluble white solid was collected by filtration under reduced pressure. The filtrate was also concentrated, and the precipitated white solid was collected by filtration under reduced pressure. Combined with the previous white solid to give 1.26 g of solid. This was suspended in 10 ml of methanol, and after suspension washing was performed for 1 hour, a white solid was collected by filtrat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com