Method for radiolabeling biological compound
A technology for biological compounds and radiolabeling, applied in the fields of radiopharmaceuticals and nuclear medicine, can solve the problems of research and clinical use of specific activity requirements, waste, low labeling yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
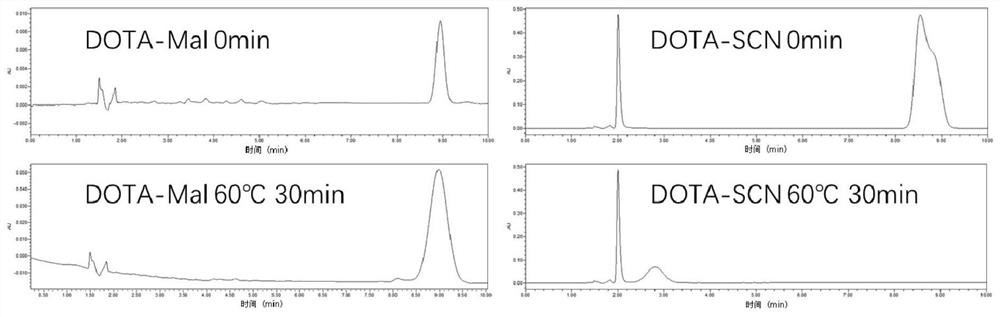
[0034] Example 1 The HPLC analysis of the stability of DOTA-Mal and DOTA-SCN
[0035]Dissolve 1mg DOTA-Mal and DOTA-SCN in 50ul DMSO respectively, then dissolve in 1ml PBS, heat at 0 time and 60°C for 30min respectively, and use high performance liquid chromatography to analyze its components (liquid phase conditions: 40% acetonitrile / water, 1ml / min), the result is as figure 1 As shown, it can be seen that DOTA-Mal can remain stable, while DOTA-SCN is less stable during heating.
Embodiment 2Ac-225
[0036] Example 2Ac-225 labeled anti-PD-L1 monoclonal antibody
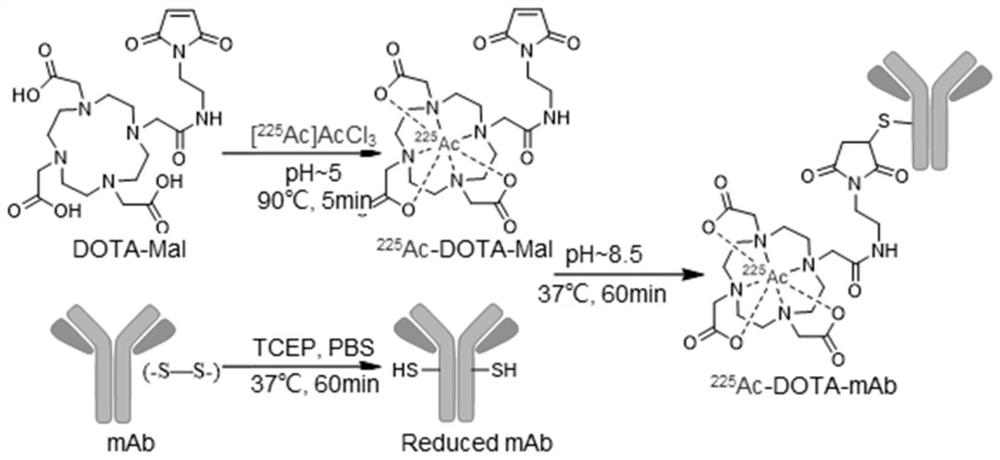
[0037] This embodiment uses DOTA-maleimide compound (Maleimido-mono-amide-DOTA, DOTA-Mal) as a bifunctional chelate, which can withstand high temperature reactions and remain stable, wherein DOTA can be combined with most common radioactive Nuclides are chelated.
[0038] The anti-PD-L1 monoclonal antibody (Adagene (Suzhou) Limited) was pretreated as follows: take 1.5 mg anti-PD-L1 monoclonal antibody, add 100 eq. TCEP to 0.1 mL PBS solution, and treat at 37 ° C for 1 h.
[0039] (1) Put 4eq. of DOTA-Mal in a 1.5ml centrifuge tube, add 10μCi[ 225 Ac]AcCl 3 Solution, and use 2M sodium acetate solution to adjust the pH to 5, and heat at 90°C for 5min.
[0040] (2) Then directly add the pretreated anti-PD-L1 monoclonal antibody to the product of step (1), and react at 37° C. for 1 h.
[0041] (3) After step (2), use PD-10 size exclusion resin (Sephadex G-25M) to purify according to its instructions, and the targe...
experiment example 1
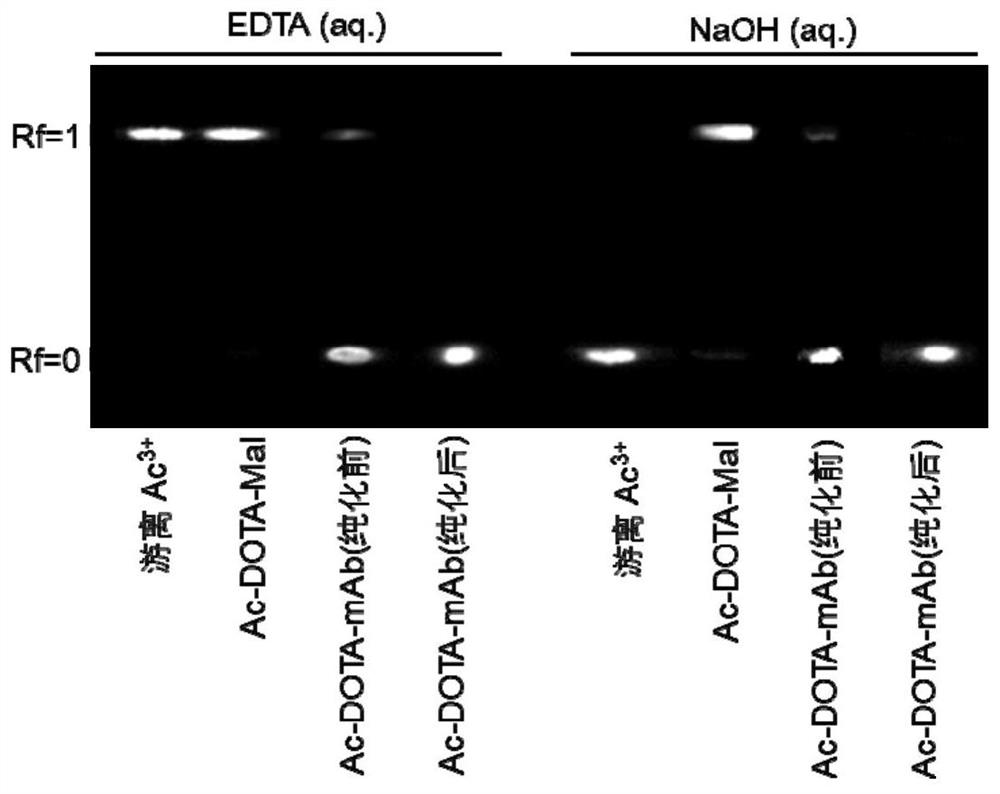
[0042] Experimental Example 1 Radioactive Thin Layer Chromatography Radiation Imaging
[0043] In order to determine the yield of radioactive labeling in Example 2, this example provides a radioactive thin-layer chromatography radiographic imaging method.
[0044] Experimental method: Take 5ul samples of the corresponding experimental group and apply them on thin-layer chromatography paper, and use the corresponding developer to develop.
[0045] Developing agent I: 10mM EDTA / water, free radioactive metal ions and radioactive ion-chelates are at the front of the solvent, and the labeled antibody is at the origin.
[0046] Developing agent II: 100mM NaOH / 9% NaCl, the radioactive ion-chelate is at the front of the solvent, and the free radioactive metal ion and the labeled antibody are at the origin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com