Ceftazidime combined powder injection and its preparation method and product specifications
A technology for ceftazidime and powder injection, which is applied in the field of ceftazidime combined powder injection and its preparation, can solve the problems of prone to allergic reaction, rapid change of impurities, poor purification effect, etc., achieves convenient clinical application, reduces white blood cell count, inhibits good bacteria effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
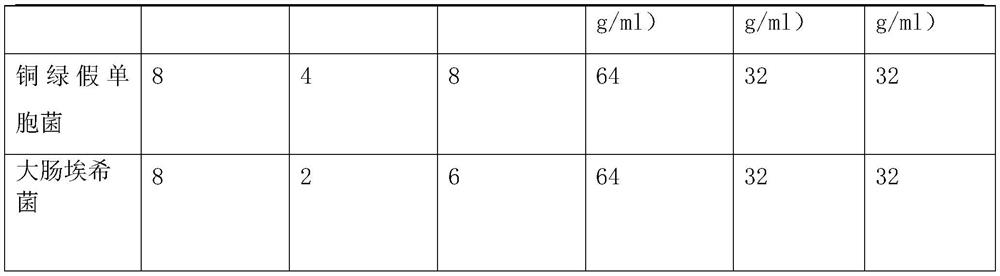
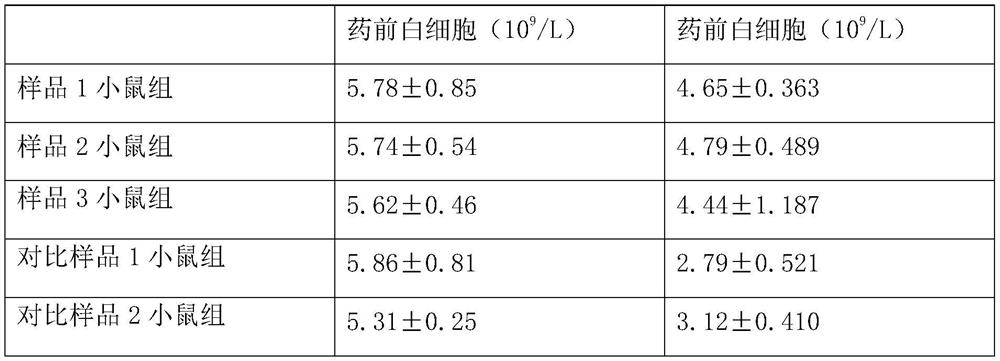
[0032] Example 1: The formula includes: 8.7 g of ceftazidime nanoparticles, 6.4 g of chitosan nanoparticles, 22 g of liposome vesicles, 0.48 g of arginine, and 5.63 g of anhydrous sodium carbonate in parts by mass. Ceftazidime nanoparticles are granulated in a nanoparticle preparation company with raw materials with a purity of 98.98%, and chitosan nanoparticles are granulated in a nanoparticle preparation company with high-purity raw materials.
[0033] Prepare samples as follows:
[0034] Including the following steps:
[0035] Step 1, preparing liposome vesicles;
[0036] Dissolve phospholipids and cholesterol in a mixed solvent of chloroform and methanol at a mass ratio of 2:1, evaporate to obtain a phospholipid film, then add PBS buffer and mannitol to the phospholipid film, and then sonicate to obtain liposome vesicles.
[0037] Step 2, preparing liposome nanoparticles;
[0038] Adding ceftazidime nanoparticles to liposome vesicles to obtain a first mixed solution, mi...
Embodiment 2
[0042] Example 2: The formula includes: 8.7 g of ceftazidime nanoparticles, 6.4 g of chitosan nanoparticles, 22 g of liposome vesicles, 0.69 g of arginine, and 3.24 g of anhydrous sodium carbonate in parts by mass. Ceftazidime nanoparticles are granulated in a nanoparticle preparation company with raw materials with a purity of 98.98%, and chitosan nanoparticles are granulated in a nanoparticle preparation company with high-purity raw materials.
[0043] Prepare samples as follows:
[0044] Including the following steps:
[0045] Step 1, preparing liposome vesicles;
[0046] Dissolve phospholipids and cholesterol in a mixed solvent of chloroform and methanol at a mass ratio of 2:1, evaporate to obtain a phospholipid film, then add PBS buffer and mannitol to the phospholipid film, and then sonicate to obtain liposome vesicles.
[0047] Step 2, preparing liposome nanoparticles;
[0048] Adding ceftazidime nanoparticles to liposome vesicles to obtain a first mixed solution, mi...
Embodiment 3
[0052] Example 3: The formula includes: 8.7 g of ceftazidime nanoparticles, 6.4 g of chitosan nanoparticles, 22 g of liposome vesicles, 1.24 g of arginine, and 5.63 g of arginine in parts by mass. Ceftazidime nanoparticles are granulated in a nanoparticle preparation company with raw materials with a purity of 98.98%, and chitosan nanoparticles are granulated in a nanoparticle preparation company with high-purity raw materials.
[0053] Prepare samples as follows:
[0054]Including the following steps:
[0055] Step 1, preparing liposome vesicles;
[0056] Dissolve phospholipids and cholesterol in a mixed solvent of chloroform and methanol at a mass ratio of 2:1, evaporate to obtain a phospholipid film, then add PBS buffer and mannitol to the phospholipid film, and then sonicate to obtain liposome vesicles.
[0057] Step 2, preparing liposome nanoparticles;
[0058] Adding ceftazidime nanoparticles to liposome vesicles to obtain a first mixed solution, mixing the first mixe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com