A kind of preparation method of battery grade ferric phosphate hydrate
A hydrated phosphoric acid, battery-level technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of limited raw material sources, iron source and phosphorus source raw material purity requirements are very high, to expand raw material sources, reduce cost The cost of auxiliary materials and the effect of reducing supersaturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
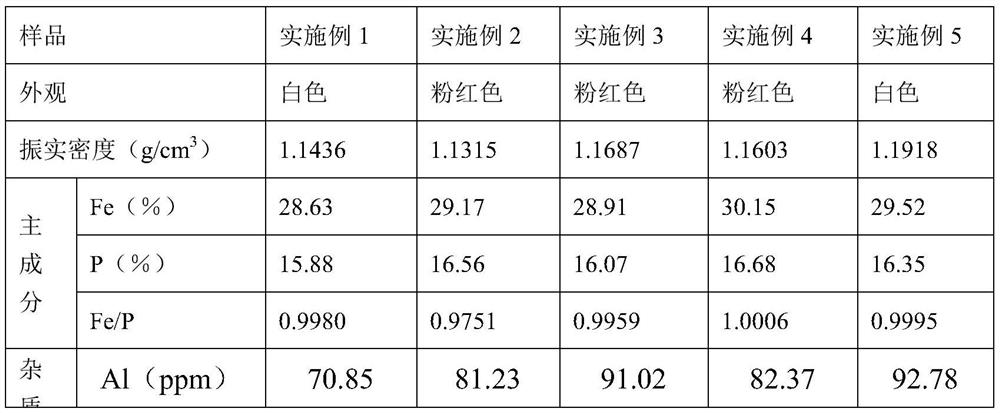
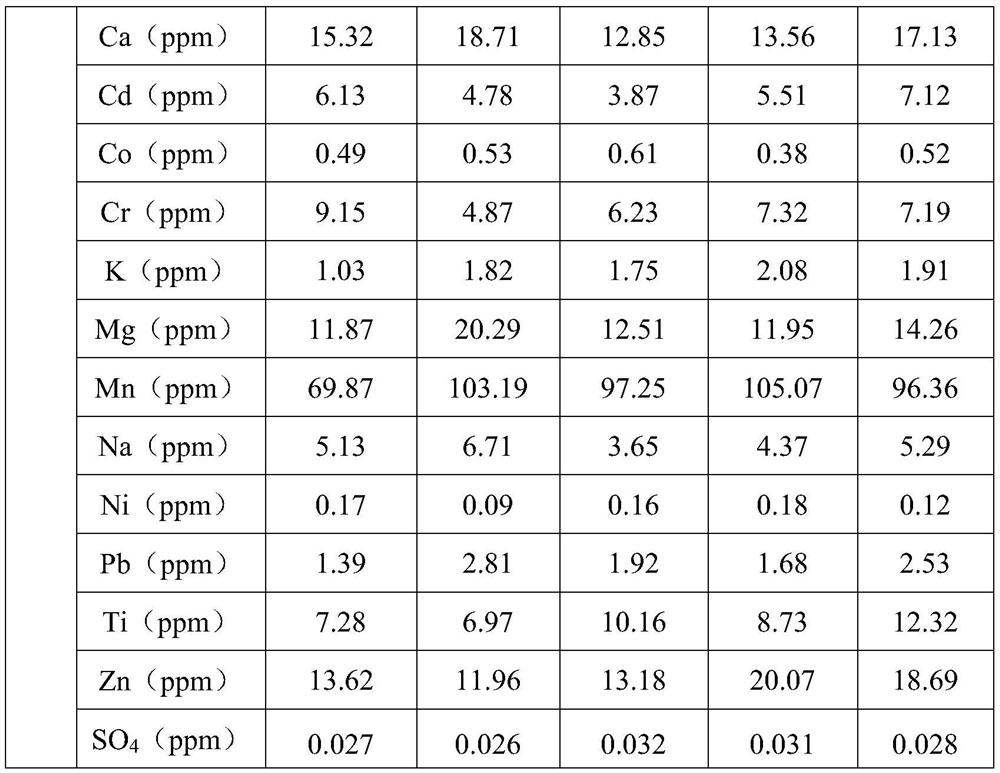
Embodiment 1
[0052] Embodiment 1 (scheme A):
[0053] Weigh 27.8g ferrous sulfate heptahydrate and dissolve it in 100ml boiled deionized water to form a 1.0mol / L ferrous sulfate solution. Weigh 13.8g ammonium dihydrogen phosphate and dissolve it in 100ml deionized water to form a 1.2mol / L solution. L ammonium dihydrogen phosphate solution, add the ammonium dihydrogen phosphate solution to the reactor containing the ferrous sulfate solution at a certain rate, after adding ammonium dihydrogen phosphate, use 1:1 dilute sulfuric acid to adjust the pH value of the solution 0.1, slowly add 1.5 times the theoretical amount of 30% hydrogen peroxide solution dropwise to oxidize ferrous iron to ferric iron, after the hydrogen peroxide is added dropwise, react at a constant temperature of 50°C for 1.0h, and then raise the temperature of the reaction system to 95°C ℃, constant temperature reflux reaction for 36.0h, the reaction is completed, vacuum filtration, washing and drying to obtain the hydrated...
Embodiment 2
[0055] Embodiment 2 (scheme A):
[0056] Measure 150ml of ferrous sulfate solution produced by dissolving high-purity iron flakes in dilute sulfuric acid, add 1:1 dilute ammonia water to adjust the pH of the solution to 4.2, heat up to 45°C, and react at a constant temperature for 1.0h to hydrolyze and purify to remove impurities such as silicon / aluminum in the solution After standing still, the ferrous sulfate purification solution was obtained by vacuum filtration, and the concentration of ferrous ions in the ferrous sulfate purification solution was measured. Take 100ml of the ferrous sulfate purification solution in a 250ml conical flask, and add 1.2 times the theoretical amount at a certain speed. Ammonium dihydrogen phosphate solution with a concentration of 1.2mol / L, after adding ammonium dihydrogen phosphate, use 1:1 dilute sulfuric acid to adjust the pH value of the solution to 0.45, and slowly add 1.2 times the theoretical amount of 30% hydrogen peroxide dropwise Sol...
Embodiment 3
[0058] Embodiment 3 (scheme B):
[0059] Weigh 278 grams of titanium dioxide by-product ferrous sulfate particles, dissolve in 1000ml pure water, stir until completely dissolved, add 1:1 dilute ammonia water, adjust the pH value of the solution to 4.5, react at a constant temperature of 50°C for 1.5h, and hydrolyze Purify and remove impurity components such as titanium / aluminum / silicon in the solution, and vacuum filter after standing for stratification to obtain ferrous sulfate purification solution. Take 100ml of the above-mentioned ferrous sulfate purification solution in a 250ml conical flask, add 1.2 times the theoretical amount of ammonium dihydrogen phosphate to the above-mentioned ferrous sulfate solution, stir until the ammonium dihydrogen phosphate is completely dissolved, and then adjust the reaction system with concentrated ammonia water pH to 8.5, the temperature was raised to 60° C. for 2.0 h at a constant temperature, and after standing for stratification, the f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com