Full-bioabsorbable composite material and application thereof, intravascular stent, and preparation methods of full-bioabsorbable composite material and intravascular stent
A technology for absorbing composite materials and vascular stents, which is applied in the field of biological materials and can solve problems such as uncontrollable degradation rate, enhanced support strength, and blocked blood vessels.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Dry polyhydroxybutyric acid at 100°C for 12 hours in advance, weigh polyhydroxybutyric acid and cellulose nanocrystals in total 300g, wherein polyhydroxybutyric acid accounts for 95wt% of the total mass percentage, and cellulose nanocrystals accounts for the total mass percentage It is 5 wt%, and the above components are mixed uniformly and then added to a twin-screw extruder for mixing, extruding and granulating.
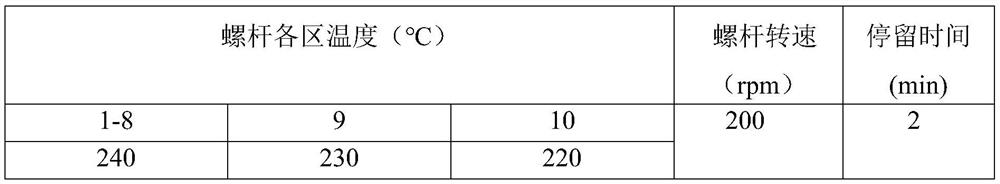
[0044] The parameters of the twin-screw extruder are set as follows:
[0045]
[0046] The pellets obtained by mixing and blending in a twin-screw extruder were dried at 100°C for 12 hours and then placed in a small injection molding machine. The pellets in the molten state are extruded into a molten mold to obtain a blood vessel stent with an outer diameter of 5 mm and a wall thickness of 0.3 mm.
[0047] Adopt polylactic acid and paclitaxel (the polylactic acid that is 95 parts by mass: the paclitaxel that is 5 parts by mass) is dissolved in chloroform...
Embodiment 2
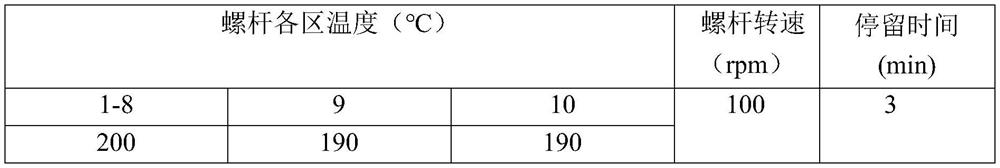
[0049] The poly-L-lactic acid was dried at 100°C for 12 hours in advance, and a total of 300 g of polyhydroxybutyric acid and starch nanocrystals were weighed. The poly-L-lactic acid accounted for 70 wt % of the total mass percentage, and the starch nano-crystals accounted for 30 wt % of the total mass percentage. After the components are mixed evenly, they are added to a twin-screw extruder for mixing, extrusion and granulation. The parameters of the twin-screw extruder are set as follows:
[0050]
[0051] The pellets obtained by mixing and blending in a twin-screw extruder were dried at 100°C for 12 hours, then placed in a small injection molding machine, and the temperature was raised to 195°C to melt. The pressure of the extrusion head was 6 MPa, and the pellets obtained by mixing were heated to a molten state. The pellets in the molten state are extruded into a molten mold for extrusion to obtain a vascular stent with an outer diameter of 7 mm and a wall thickness of ...
Embodiment 3
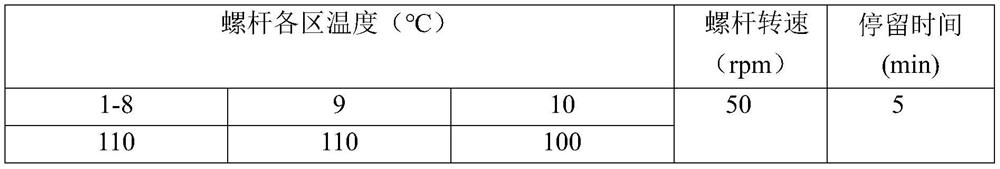
[0054] Dry polycaprolactone at 40° C. for 12 hours in advance, weigh polycaprolactone and chitosan nanocrystals in total 300 g, polycaprolactone accounts for 80 wt % of the total mass percentage, and starch nano crystals accounts for 20 wt % of the total mass %, the above components are mixed uniformly and then added to a twin-screw extruder for mixing, extrusion and granulation. The parameters of the twin-screw extruder are set as follows:
[0055] The parameters of the twin-screw extruder are set as follows:
[0056]
[0057] The pellets obtained by mixing and blending in a twin-screw extruder were dried at 40°C for 12 hours, then placed in a small injection molding machine, and the temperature was raised to 90°C to melt. The pressure of the extrusion head was 1 MPa, and the pellets obtained by mixing were heated to a molten state. The pellets in the molten state are extruded into a molten mold for extrusion to obtain a blood vessel stent with an outer diameter of 20 mm ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com