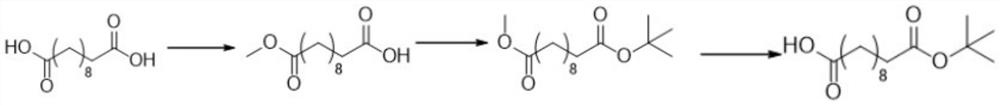
Synthetic method of octadecanedioic acid mono-tert-butyl ester
A technology of octadecanedicarboxylic acid and mono-tert-butyl ester, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds. It can solve difficult high-purity target products, limit large-scale production, and limit applications. and other problems, to achieve high reaction conversion rate, enhanced ability to capture protons or electrons, and unabated catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 18
[0020] The synthetic method of embodiment 1 octadecane dicarboxylic acid mono-tert-butyl ester
[0021] (1) Add sebacic acid and methanol with a molar ratio of 1:1 into the reactor, under the catalysis of concentrated sulfuric acid, heat to 75-80°C, reflux for esterification, cool, separate and purify to obtain octadecanedicarboxylic acid Monomethyl ester; the mass concentration of concentrated sulfuric acid is 65%wt, and the add-on of concentrated sulfuric acid is 2.5wt% of sebacic acid;
[0022] (2) Dissolve octadecanedicarboxylic acid monomethyl ester and isobutylene with a molar ratio of 1:1.2 in an organic solvent, add an appropriate amount of composite catalyst at -20°C to 0°C, and stir the reaction for 3-5 hours , remove the composite catalyst, and concentrate the reaction solution to obtain a concentrate; the mass ratio of the composite catalyst to sebacic acid is 1:2;
[0023] Wherein, the composite catalyst is prepared by the following method: bismuth nitrate soluti...
Embodiment 2 18
[0025] The synthetic method of embodiment 2 octadecane dicarboxylic acid mono-tert-butyl esters
[0026] (1) Add sebacic acid and methanol with a molar ratio of 1:1.2 into the reactor, under the catalysis of concentrated sulfuric acid, heat to 75-80°C, reflux for esterification, cool, separate and purify to obtain octadecanedicarboxylic acid Monomethyl ester; the mass concentration of concentrated sulfuric acid is 75%wt, and the add-on of concentrated sulfuric acid is 3.5wt% of sebacic acid;
[0027] (2) Dissolve octadecanedicarboxylic acid monomethyl ester and isobutylene with a molar ratio of 1:1.5 in an organic solvent, add an appropriate amount of composite catalyst at -20°C to 0°C, and stir for 3-5 hours. , remove the composite catalyst, and concentrate the reaction solution to obtain a concentrate; the mass ratio of the composite catalyst to sebacic acid is 1:4;
[0028] Wherein, the composite catalyst is prepared by the following method: bismuth nitrate solution, citri...
Embodiment 3 18
[0030] The synthetic method of embodiment 3 octadecane dicarboxylic acid mono-tert-butyl esters
[0031] (1) Add sebacic acid and methanol with a molar ratio of 1:1.1 into the reactor, under the catalysis of concentrated sulfuric acid, heat to 75-80°C, reflux for esterification, cool, separate and purify to obtain octadecanedicarboxylic acid Monomethyl ester; the mass concentration of concentrated sulfuric acid is 70%wt, and the added amount of concentrated sulfuric acid is 3wt% of sebacic acid;
[0032] (2) Dissolve octadecanedicarboxylic acid monomethyl ester and isobutylene with a molar ratio of 1:1.4 in an organic solvent, add an appropriate amount of composite catalyst at -20°C to 0°C, and stir the reaction for 3-5 hours , remove the composite catalyst, and concentrate the reaction solution to obtain a concentrate; the mass ratio of the composite catalyst to sebacic acid is 1:3;
[0033] Wherein, the composite catalyst is prepared by the following method: bismuth nitrate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com