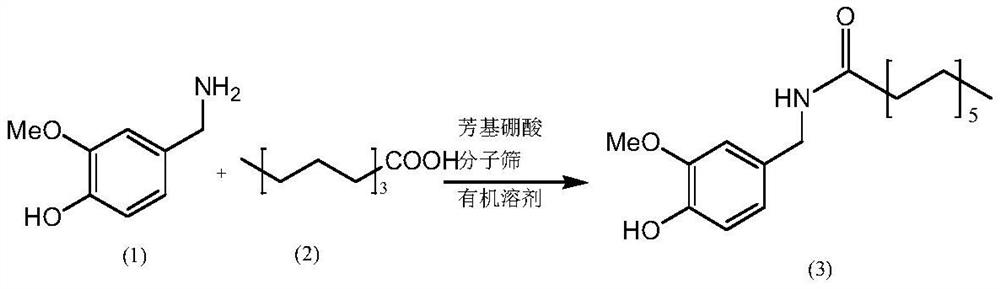
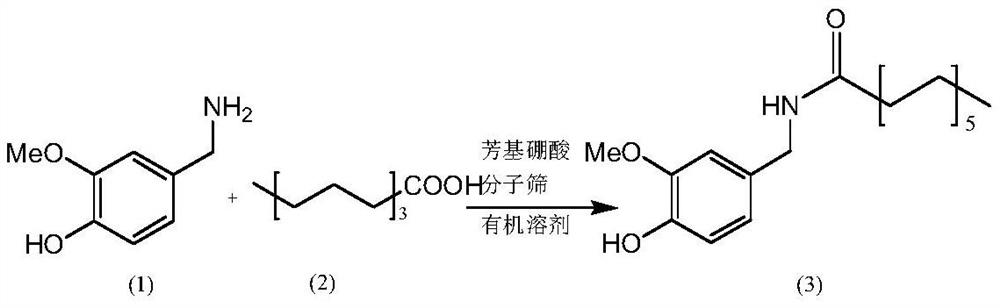
Method for preparing nonivamide
A technology for vanillamide and n-nonanoic acid, applied in the field of preparing vanillamide n-nonanoic acid, can solve the problems of low yield, unenvironmental protection, complicated post-processing and the like, achieve simple post-processing, avoid the use of organic solvents, and avoid sodium salts The effect of pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 aryl boronic acid
[0031] The aryl boronic acid of the present invention is prepared by a prior art method: Synthesis, evaluation and application of novel bifunctional N, N-diisopropylbenzylamine boronic acid catalysts for direct amide formation between carboxylic acids and amines, Green Chemistry, 2008.
Embodiment 2
[0033] Add 2-((N,N-diisopropyl)methyl)phenylboronic acid (0.0327mol, 10mol%) to 200ml methylene chloride in a 500ml three-necked flask, add active Stir with molecular sieves, add 50g (0.327mol) of 3-methoxy-4-hydroxybenzylamine at room temperature, stir and heat to reflux at 40°C, add 43g (0.272mol) of n-nonanoic acid dropwise through the dropping funnel, and keep stirring at reflux for 0.5h After the reaction, cool down to room temperature, add 100ml of water and stir for 5 minutes, then let stand to separate layers, take the oily layer, and distill under reduced pressure at 30°C to recover dichloromethane, stop the reduced-pressure distillation after obtaining a light yellow transparent liquid, cool the remaining liquid to room temperature, and forcefully After stirring for 30 min, 79.6 g of white solid powder was precipitated, with a yield of 95.8% and a purity of 99.3% by HPLC.
[0034] White powder solid analysis
[0035] NMR spectrum characterization: 1H NMR (CDCl3, TM...
Embodiment 3
[0039] Add 2-((N,N-dimethyl)methyl)phenylboronic acid (0.0327mol, 10mol%) to 200ml toluene in a 500ml three-necked flask, add active Stir with molecular sieves, add 50g (0.327mol) of 3-methoxy-4-hydroxybenzylamine at room temperature, stir and heat to reflux at 110°C, add 43g (0.272mol) of n-nonanoic acid dropwise through the dropping funnel, and keep stirring at reflux for 2.5h After the reaction, cool down to room temperature, add 100ml of water and stir for 5 minutes, then let stand to separate layers, take the oily layer, and recover toluene by vacuum distillation at 60°C, stop the vacuum distillation after obtaining a light yellow transparent liquid, cool the remaining liquid to room temperature, and stir vigorously for 30 minutes , 78.9 g of white solid powder was precipitated, the yield was 95.0%, and the HPLC purity was 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com