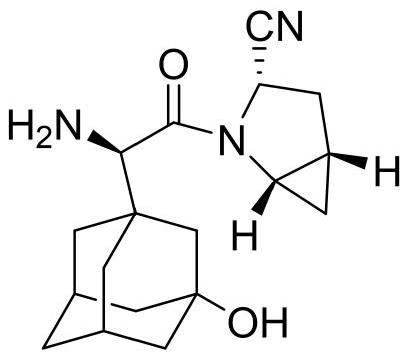
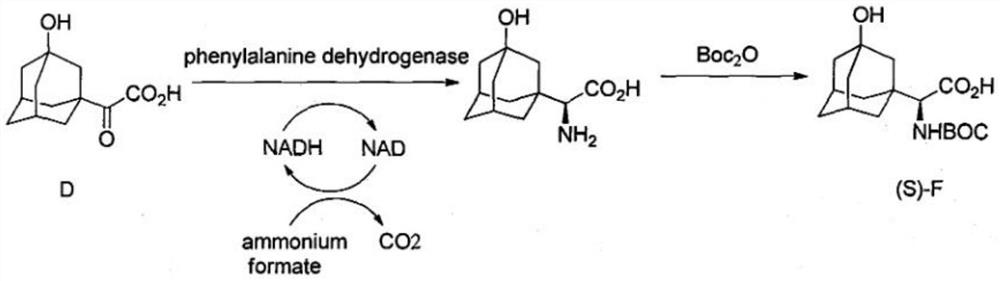
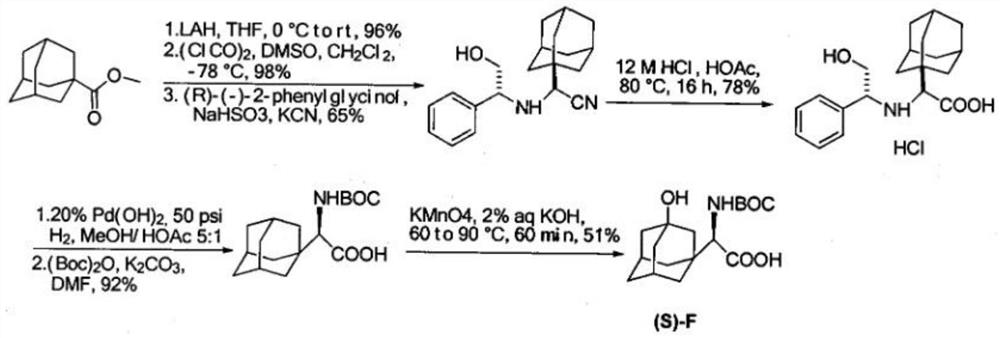
Synthetic method of saxagliptin intermediate
A synthesis method and intermediate technology, applied in the field of synthesis of saxagliptin intermediates, can solve the problems of dangerous operation, expensive raw materials, environmental pollution, etc., and achieve the effects of short route, reduced production cost and stable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of 1,3-dibromoadamantane (compound 2):
[0035] Get adamantane (13.6g, 100mmol), aluminum tribromide (58.6g, 220mmol) and boron tribromide (7.5g, 30mmol) and join in the reactor, add the liquid bromine of 109mL under the condition of 0 ℃, and Stir at 0°C for 30 minutes, then raise the temperature to 70°C and react for 48 hours. After the reaction, cool to room temperature, add saturated sodium bisulfite to quench the reaction, filter and wash with deionized water to obtain 23.0 g of yellow solid 1,3-di Bromoadamantane, the yield is 78%.
[0036] 1 H-NMR (400MHz, DMSO-d 6 ):δ2.83(s,1H),2.73(s,1H),2.48(s,4H),2.35-2.21(m,8H).
Embodiment 2
[0038] Preparation of 3-bromo-1-adamantyl-D-glycine (compound 3):
[0039] Add sodium (1.7g, 74.8mmol) to 500mL of petroleum ether, add diethyl acetamidomalonate (16.2g, 74.8mmol) while stirring, and stir at room temperature. After the metal sodium is completely dissolved, add Compound 2 (20.0 g, 68 mmol), and reacted at room temperature for 12 hours. After the reaction, 200ml of distilled water was added, the organic layer was separated, the solvent was concentrated, and L-arginine (17.8g, 102mmol) was dissolved in a mixed solvent of 7.5mL of concentrated sulfuric acid, 22.5mL of distilled water and 75mL of glacial acetic acid to form L- The acidic solution of arginine, then the acidic solution of L-arginine was added to the reaction after the concentrated solvent, stirred and refluxed for 6 hours, cooled to room temperature after the reaction and poured into ice water, extracted with dichloromethane, combined organic layer, the solvent was evaporated, and the residue was re...
Embodiment 3
[0042] Preparation of 3-bromo-1-adamantyl-D-glycine (compound 4):
[0043] Dissolve KOH (32.0g, 57.2mmol) in 200mL of water, add compound 3 (15g, 52.0mmol), and reflux for 8h under stirring. After the reaction, extract and concentrate and recrystallize to obtain 9.6g of white solid with a yield of 82%. The ee value is 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com