A kind of compound and its preparation method and application
A compound, methyl phenyl technology, applied in the field of compound and its preparation, achieves the effect of simple synthesis process, good inhibitory activity and easy recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
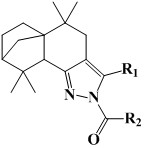
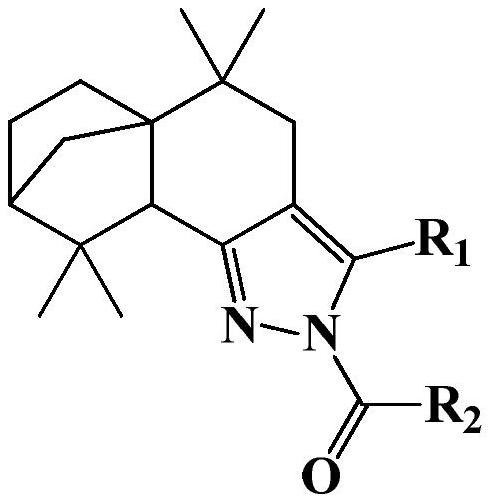
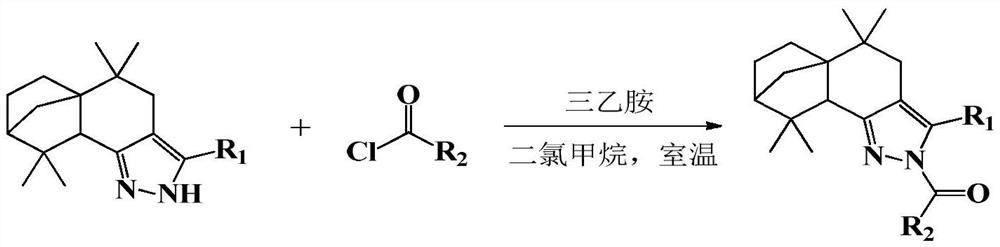
[0024] 2-(4′-fluorobenzoyl)-3-(4′-fluorophenyl)-5,5,9,9-tetramethyl-4,5,5a,6,7,8,9,9a - Preparation of octahydro-5a,8-methanobenzo[g]indazole (compound 1):
[0025]
[0026] Add 3-(4′-fluorophenyl)-5,5,9,9-tetramethyl-4,5,5a,6,7,8,9,9a-octahydro- 5a,8-Methylenebenzo[g]indazole (6mmol), triethylamine (9mmol) and anhydrous DCM (20mL), add p-fluorobenzoyl chloride (8mmol) dropwise at room temperature, after the addition Reacted at room temperature for 6 hours, and GC detected that the reaction was complete; after the reaction was completed, 30 mL of distilled water was added, extracted 3 times with 30 mL of ethyl acetate, the organic layers were combined, concentrated, and recrystallized from ethyl acetate / petroleum ether to obtain a yellow solid powder 2-(4 '-Fluorobenzoyl)-3-(4'-fluorophenyl)-5,5,9,9-tetramethyl-4,5,5a,6,7,8,9,9a-octahydro- 5a,8-Methylene benzo[g]indazole, the yield is 69.5%. 1 H NMR (600MHz, Chloroform-d) δ8.09(d, J=8.8, 5.5Hz, 2H), 7.33(d, J=8.6, 5.4Hz,...
Embodiment 2
[0028] 2-(4′-fluorobenzoyl)-3-(4′-chlorophenyl)-5,5,9,9-tetramethyl-4,5,5a,6,7,8,9,9a - Preparation of octahydro-5a,8-methanobenzo[g]indazole (compound 2):
[0029]
[0030] The preparation method is the same as in Example 1, wherein, 3-(4'-chlorophenyl)-5,5,9,9-tetramethyl-4,5,5a,6,7,8,9,9a-eight Hydrogen-5a,8-methanobenzo[g]indazole in place of 3-(4′-fluorophenyl)-5,5,9,9-tetramethyl-4,5,5a,6,7 ,8,9,9a-octahydro-5a,8-methanobenzo[g]indazole, product 2-(4′-fluorobenzoyl)-3-(4′-chlorophenyl)- 5,5,9,9-tetramethyl-4,5,5a,6,7,8,9,9a-octahydro-5a,8-methanobenzo[g]indazole as yellow solid powder , yield 72.8%. 1 H NMR (600MHz, Chloroform-d) δ: 8.11 (dd, J = 8.9, 5.4Hz, 2H), 7.40 (d, J = 8.5Hz, 2H), 7.29 (d, J = 8.5Hz, 2H), 7.13 (t,J=8.7Hz,2H),2.47(d,J=15.8Hz,1H),2.37(s,1H),2.15(d,J=15.9Hz,1H),1.88–1.92(m,1H) ,1.81(s,1H),1.73–1.78(m,1H),1.64(d,J=10.0Hz,1H),1.50–1.56(m,1H),1.27(d,J=10.0Hz,1H), 1.24(s,3H),1.17–1.22(m,1H),1.05(s,3H),0.88(s,3H),0.77(s,3H); HR-MS (ESI + )m / z: c...
Embodiment 3
[0032] 2-(4′-fluorobenzoyl)-3-(4′-methylphenyl)-5,5,9,9-tetramethyl-4,5,5a,6,7,8,9, Preparation of 9a-octahydro-5a,8-methanobenzo[g]indazole (compound 3):
[0033]
[0034]The preparation method is the same as in Example 1, wherein, 3-(4'-methylphenyl)-5,5,9,9-tetramethyl-4,5,5a,6,7,8,9,9a- Octahydro-5a,8-methanobenzo[g]indazole replacing 3-(4′-fluorophenyl)-5,5,9,9-tetramethyl-4,5,5a,6, 7,8,9,9a-octahydro-5a,8-methanobenzo[g]indazole, product 2-(4′-fluorobenzoyl)-3-(4′-methylphenyl )-5,5,9,9-tetramethyl-4,5,5a,6,7,8,9,9a-octahydro-5a,8-methanobenzo[g]indazole is yellow Solid powder, yield 67.8%. 1 H NMR (600MHz, Chloroform-d) δ: 8.11 (dd, J = 8.8, 5.6Hz, 2H), 7.24 (d, J = 3.3Hz, 4H), 7.12 (t, J = 8.7Hz, 2H), 2.48 (d,J=15.8Hz,1H),2.40(s,3H),2.37(s,1H),2.20(d,J=15.8Hz,1H),1.88–1.94(m,1H),1.80(s, 1H),1.74–1.78(m,1H),1.66(d,J=9.9Hz,1H),1.50–1.56(m,1H),1.27(d,J=9.9Hz,1H),1.24(s,3H ),1.17–1.22(m,1H),1.04(s,3H),0.89(s,3H),0.78(s,3H); HR-MS (ESI + )m / z: calculated for C 30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com