Glucagon-like peptide-1 analogue monomer, glucagon-like peptide-1 analogue dimer, and application of glucagon-like peptide-1 analogue monomer and glucagon-like peptide-1 analogue dimer
A technology for glucagon and analogues, applied in the direction of glucagon, hormone peptides, biochemical equipment and methods, etc., can solve the problems of complex and harsh purification conditions, complex tertiary structure, and limited clinical application, and achieve therapeutic Better effect, simple process, and simple peptide structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
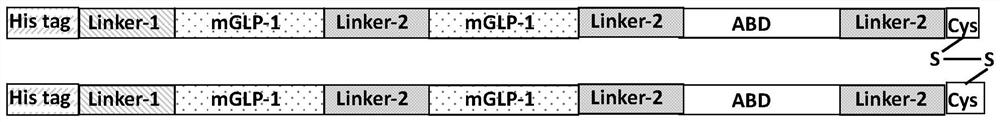
[0076] According to the metabolism characteristics of GLP-1 in vivo, the present invention has modified it from the following aspects: under the premise of ensuring the biological activity of GLP-1, the key amino acid at the action site of the mutated protease DPP-4 is replaced by Gly Ala at position 30 and Arg at position 30, the mutant mGLP-1 of GLP-1 is obtained; mGLP-1 is tandemly fused with the serum albumin binding domain ABD to increase molecular weight and reduce renal clearance, while the fusion protein passes through ABD Non-covalently binds to serum albumin HSA to form a complex, utilizes the FcRn-mediated recycling mechanism, and evades lysosome and glomerular filtration.
[0077] In the present invention, in order to facilitate the purification of the expressed fusion protein, a His purification tag is designed at the N-terminus; in order to release the active fusion protein, the restriction site Linker-1 of endogenous protease thrombin and DPP-4 is designed to Re...
Embodiment 2
[0090] Using the glucagon-like peptide-1 analog monomer in Example 1 to construct a glucagon-like peptide-1 analog dimer, the specific process includes the following:
[0091] 1. pET30a-m(GLP-1) 2 -Construction of ABD recombinant plasmid
[0092] pET30a-m(GLP-1) 2 -The construction of the ABD recombinant plasmid comprises four steps:
[0093] 1. SOE-PCR amplification (mGLP-1) 2 -ABD fusion gene
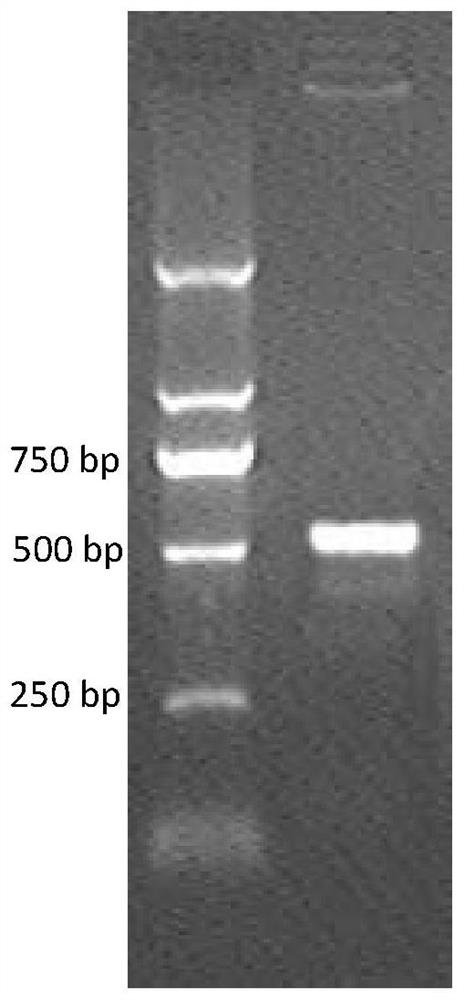
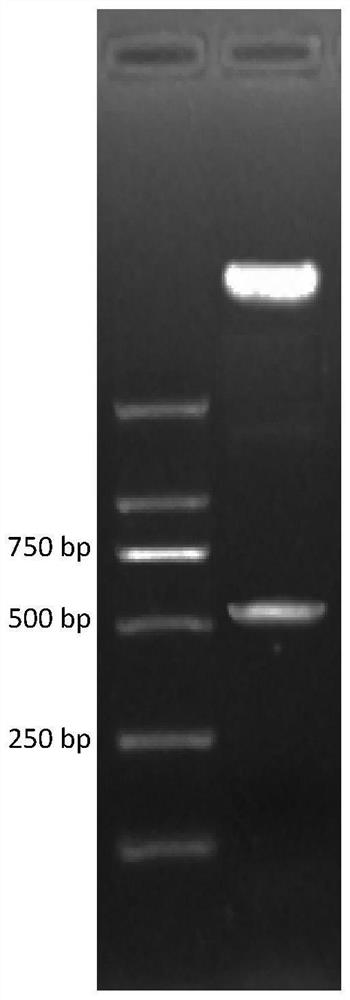
[0094] (mGLP-1) 2 - The ABD fusion gene has 507bp, and the full sequence of the fusion gene was obtained by three-step SOE-PCR using 3 pairs of overlapping complementary primers.
[0095] (1) The first step SOE-PCR
[0096] According to (mGLP-1) 2 -The amino acid sequence of the ABD fusion protein, using Escherichia coli preferred codons, designing overlapping complementary primers P1 and P2, the primer sequences are as follows:
[0097] Upstream primer P1 (SEQ ID NO.3)
[0098]
[0099] Downstream primer P2 (SEQ ID NO.4)
[0100] 5'-AACGTCGGAAGTGAAGGTACCTTCACCGTGAGCGGAGC...
Embodiment 3
[0147] 3.1. Glucagon-like peptide-1 monomer m (GLP-1) 2 -ABD and its dimer [m(GLP-1) 2 -ABD] 2 Pharmacokinetics in SD rats
[0148] SD rats with a body weight of about 300 g (provided by Suibei Experimental Animal Farm, Baiyun District, Guangzhou City) were randomly divided into two groups (m(GLP-1) 2 -ABD group, [m(GLP-1) 2 -ABD] 2 group), 8 rats in each group, half male and half male. The concentration of 100nmol / mL (m(GLP-1) 2 -ABD solution, [m(GLP-1) 2 -ABD] 2 Solution (prepared and obtained in Example 2). The dose of 30nmol / kg was subcutaneously injected into the rats of each group respectively. Blood was collected from the orbital venous plexus, and blood samples were collected 0 h before administration and 1, 2, 3, 4, 5, 6, 8, 10, 12, 16, 24, 36, and 48 h after administration, and placed in EDTA-treated EP tube. 4°C, 4000g, centrifuge at low temperature for 10min, take the supernatant, quickly freeze it in liquid nitrogen and store it at -80°C for later use, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com