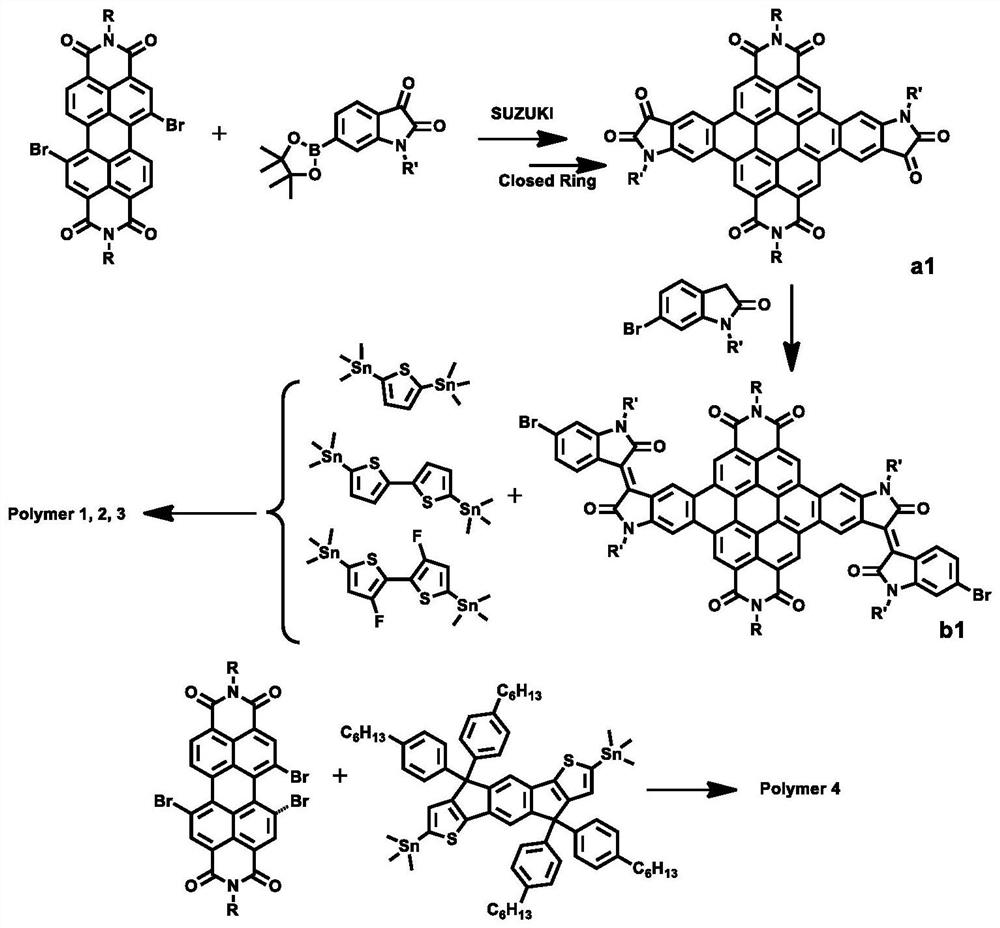
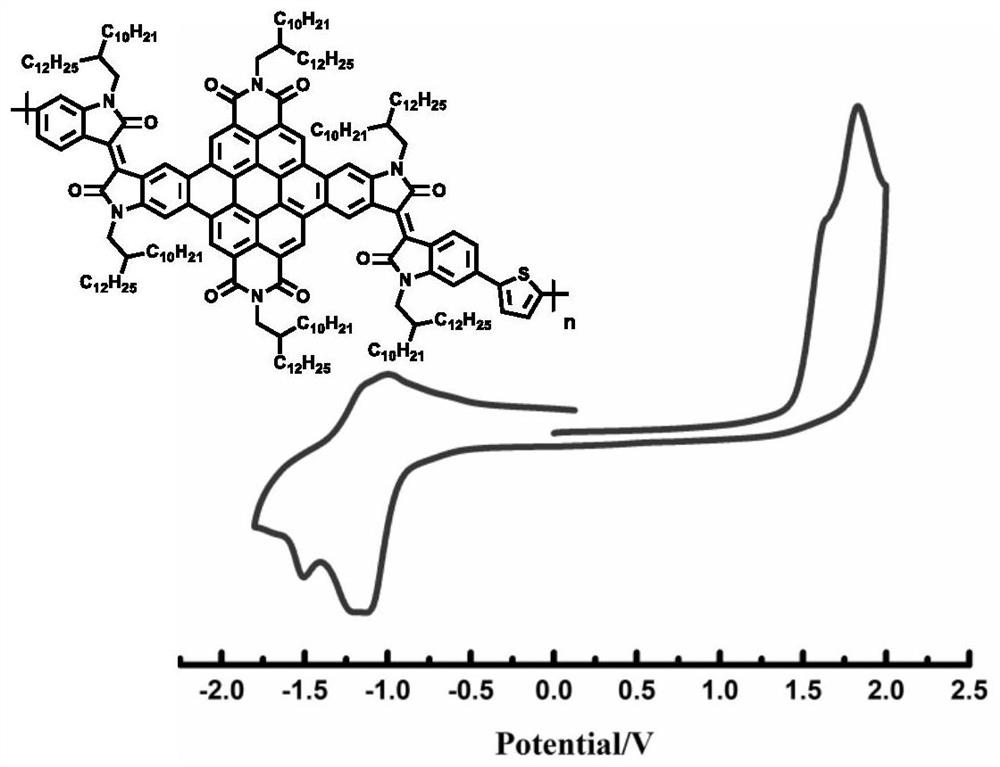
D-A polymer based on conjugate plane expansion of 3,4,9,10-perylenetetracarboxylic diimide and preparation method thereof
A technology of perylene imide and conjugated plane, which is applied in the field of D-A polymer and its preparation based on perylene imide conjugated plane expansion, can solve the problems of systematic research, fusion of building units, etc., and achieve high electron affinity The effect of energy, low lowest unoccupied molecular orbital
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
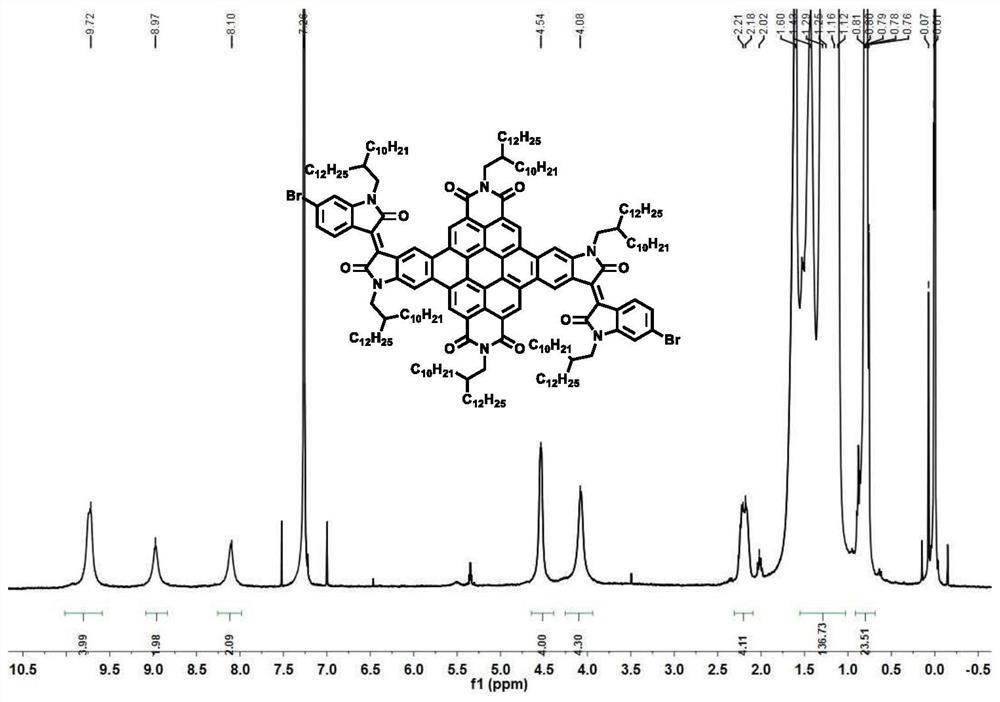
[0060] (1) Substitute 0.76g of 2-decyltetradecane-1-amine substituted 1,7-dibromoperyleneimide (DT-PDI) and 0.84g of 1-bromo-2-decyltetradecane Add the 6-boronester isatin into the sealed tube, degas and ventilate, three times in a row; then add 20ML of tetrahydrofuran solvent and 1.5ML (2mol.L) of tripotassium phosphate aqueous solution and stir, and add 17.2mg of Tris(dibenzylideneacetone) dipalladium catalyst and 21.8 mg of tri-tert-butylphosphine tetrafluoroborate were pumped and ventilated three times, and then the temperature was raised to 80°C for 12 hours. Purified by chromatographic column, the ratio of eluent dichloromethane to petroleum ether is 3:5; weigh 50 mg of the obtained crude product into a column reaction flask, add a grain of iodine as a catalyst, and then add 20 mL Toluene solvent, heated at 90°C, under the atmosphere of air, LED blue light continued to irradiate for 12 hours, return to room temperature after the reaction, distill the reaction solution un...
Embodiment 2
[0065] (1) Substitute 0.76g of 2-decyltetradecane-1-amine substituted 1,7-dibromoperyleneimide (DT-PDI) and 0.84g of 1-bromo-2-decyltetradecane Add the 6-boronester isatin into the sealed tube, degas and ventilate, three times in a row; then add 20ML of tetrahydrofuran solvent and 1.5ML (2mol.L) of tripotassium phosphate aqueous solution and stir, and add 17.2mg of Tris(dibenzylideneacetone) dipalladium catalyst and 21.8 mg of tri-tert-butylphosphine tetrafluoroborate were pumped and ventilated three times, and then the temperature was raised to 80°C for 12 hours. Purified by chromatographic column, the ratio of eluent dichloromethane to petroleum ether is 3:5; weigh 50 mg of the obtained crude product into a column reaction flask, add a grain of iodine as a catalyst, and then add 20 mL Toluene solvent, heated at 90°C, under the atmosphere of air, LED blue light continued to irradiate for 12 hours, return to room temperature after the reaction, distill the reaction solution un...
Embodiment 3
[0070] (1) Substitute 0.76g of 2-decyltetradecane-1-amine substituted 1,7-dibromoperyleneimide (DT-PDI) and 0.84g of 1-bromo-2-decyltetradecane Add the 6-boronester isatin into the sealed tube, degas and ventilate, three times in a row; then add 20ML of tetrahydrofuran solvent and 1.5ML (2mol.L) of tripotassium phosphate aqueous solution and stir, and add 17.2mg of Tris(dibenzylideneacetone) dipalladium catalyst and 21.8 mg of tri-tert-butylphosphine tetrafluoroborate were pumped and ventilated three times, and then the temperature was raised to 80°C for 12 hours. Purified by chromatographic column, the ratio of eluent dichloromethane to petroleum ether is 3:5; weigh 50 mg of the obtained crude product into a column reaction flask, add a grain of iodine as a catalyst, and then add 20 mL Toluene solvent, heated at 90°C, under the atmosphere of air, LED blue light continued to irradiate for 12 hours, return to room temperature after the reaction, distill the reaction solution un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com