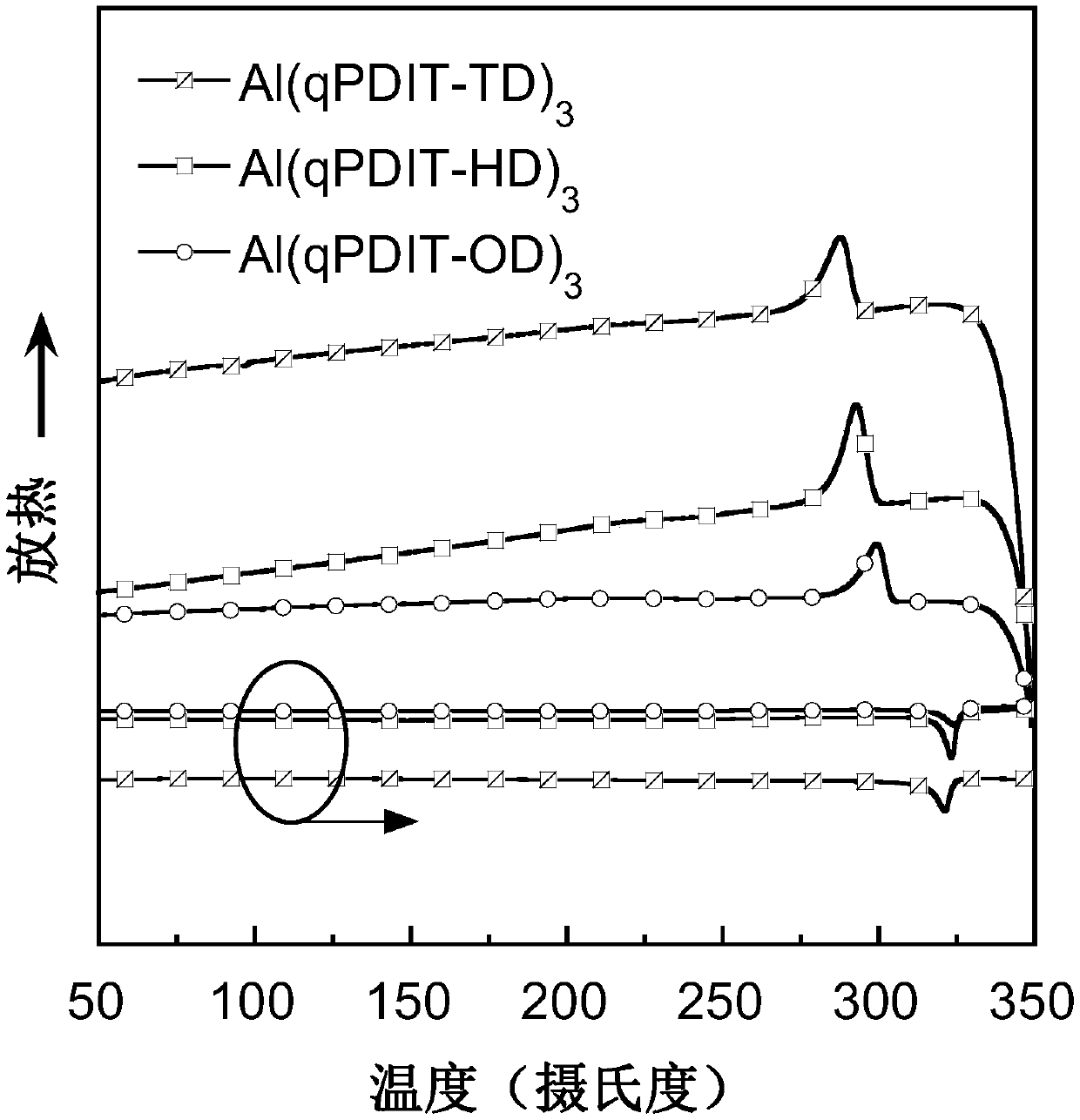
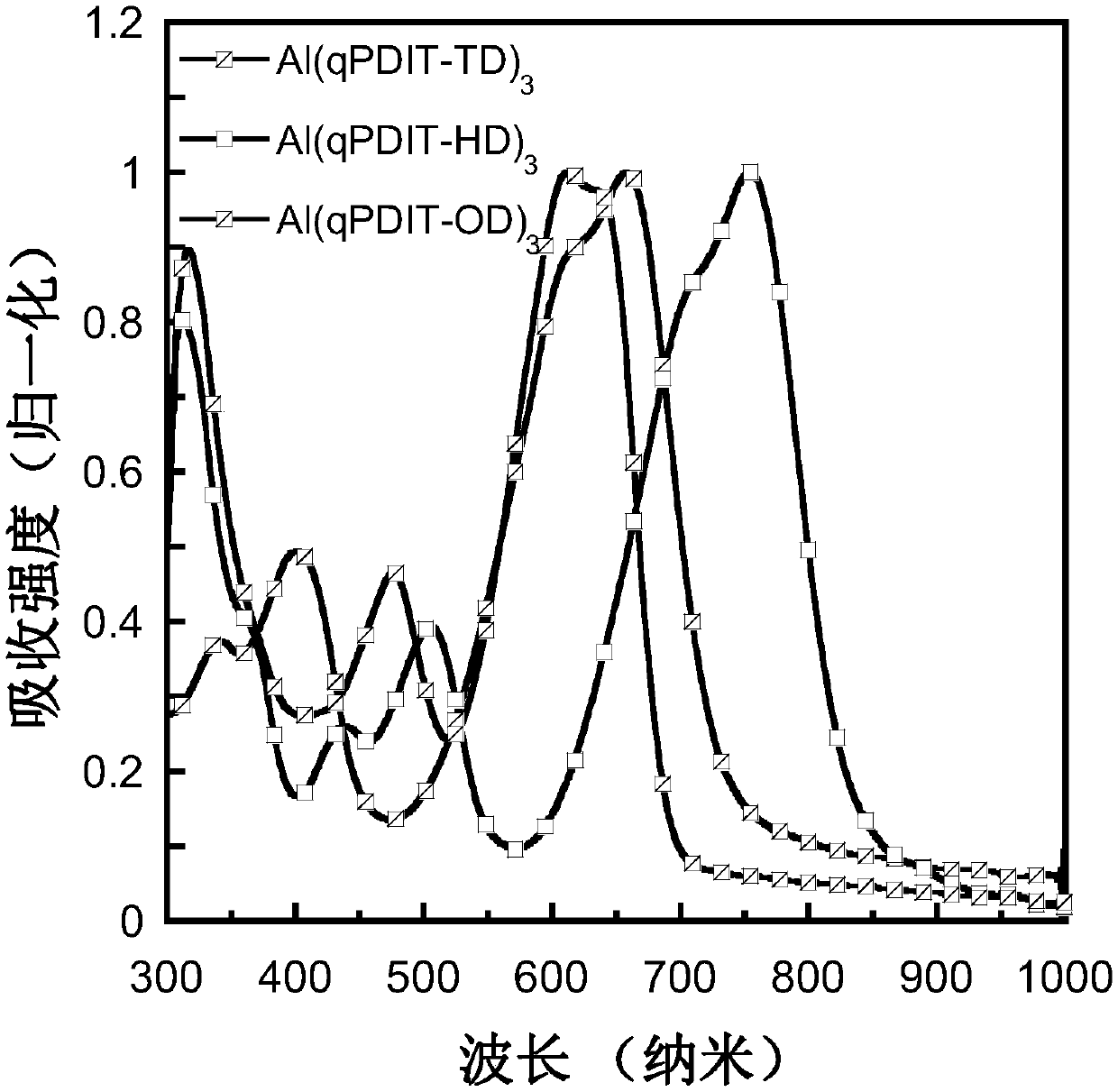
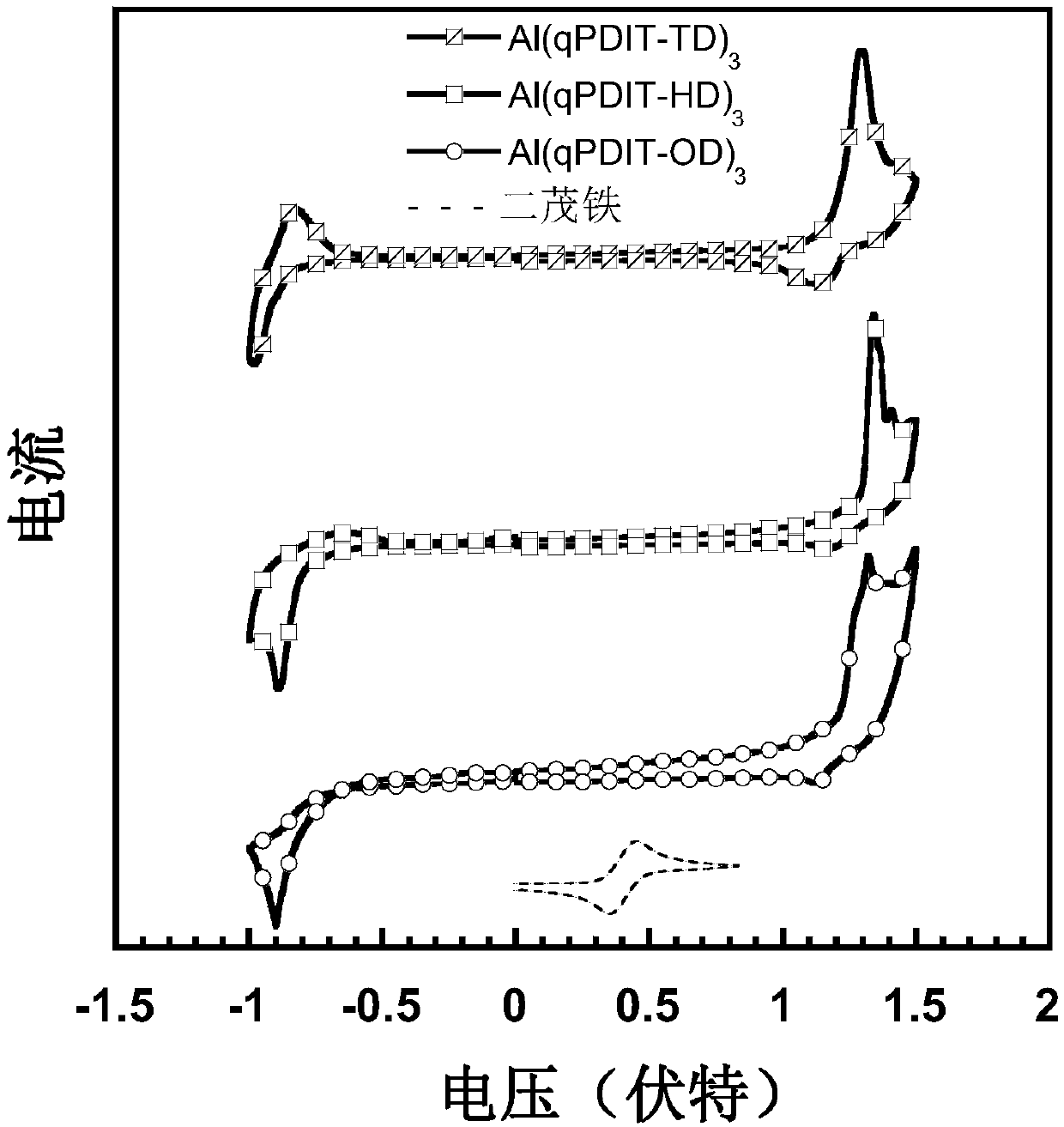
Hydroxyquinolinyl metal organic small molecule cooperation compound material grafted with perylene diimide and aromatic groups, preparation method and applications thereof
A technology of grafting perylene diimide and perylene diimide, which is applied in organic chemistry, semiconductor/solid-state device manufacturing, electric solid-state device, etc., to achieve good solubility, improve electron affinity, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of 5-(3-hexyl-5-(trimethyltin)thiophen-2-yl)-8-hydroxyquinoline (F)
[0032] (1) Under nitrogen atmosphere, add compound A (7-fluoro-8-hydroxyquinoline) (1.5g, 10.0mmol), iron powder (10.0mg, 0.18mmol) and 50mL of chloroform into a 150mL three-necked flask, Cool in an ice-water bath; add 5 mL of liquid bromine (3.3 g, 21.0 mmol) / chloroform mixed solution dropwise, and the temperature in the bottle does not exceed 5°C during the dropwise addition. After the reaction was completed, it was filtered and recrystallized from chloroform to obtain light yellow solid B (5-bromo-7-fluoro-8-hydroxyquinoline) (1.6 g, yield 72%).
[0033]
[0034] (2) Under nitrogen atmosphere, add compound B (1.3g, 6.0mmol), compound C (2.0g, 6.0mmol), catalyst Pd (PPh 3 ) 4 (0.19g, 0.17mmol), then add 100mL tetrahydrofuran as a solvent, and react at 105°C for 12h. Post-treatment: when the reaction mixture is put into a single-necked bottle, it is mixed with silica gel powder for ...
Embodiment 2
[0041] 5-(2-(N,N'-bis(n-tridecyl)-1,4,5,8-tetraketoneperylenediimide)-4-hexylthiophene)-7-fluoro-8-hydroxyl Preparation of quinoline (M1)
[0042] (1) Under nitrogen atmosphere, add compound F (1.19g, 2.5mmol) and compound G (N,N'-bis(n-tridecyl)-2-bromo-1,4,5 , 8-tetraketone perylene diimide) (2.08g, 2.5mmol), catalyst Pd (PPh 3 ) 4 (0.06g, 0.05mmol), then added toluene (100mL), heated to 105°C for 12 hours. Post-treatment: when the reaction mixture is put into a single-necked bottle, it is mixed with silica gel powder for rotary evaporation, and then purified through a column with a silica gel column (the eluent is selected petroleum ether: dichloromethane is 2:1 (v / v)), to obtain Yellow solid compound H (5-(2-(N,N'-bis(2-octyldodecyl)-1,4,5,8-tetraketoneperylenediimide)-4-hexylthiophene) -7-fluoro-8-hydroxyquinoline) (2.00 g, 75% yield).
[0043]
[0044] (2) Under nitrogen atmosphere, add compound H (1.60g, 1.5mmol) and I to a 250mL two-necked bottle 2 (0.46g, 1.8...
Embodiment 3
[0047] 5-(2-(N,N'-bis(2-hexyldecyl)-1,4,5,8-tetraketone perylenediimide)-4-hexylthiophene)-7-fluoro-8-hydroxyl Preparation of quinoline (M2)
[0048] (1) Under nitrogen atmosphere, add compound F (1.19g, 2.5mmol) and compound I (N,N'-bis(2-hexyldecyl)-2-bromo-1,4,5 , 8-tetraketone perylene diimide) (2.30g, 2.5mmol), catalyst Pd (PPh 3 ) 4 (0.06g, 0.05mmol), then added toluene (100mL), heated to 105°C for 12 hours. Post-treatment: when the reaction mixture is put into a single-necked bottle, it is mixed with silica gel powder for rotary evaporation, and then the column is purified with a silica gel column (the eluent is selected petroleum ether: dichloromethane is 2:1 (v / v)), Compound J (5-(2-(N,N'-bis(2-octyldodecyl)-1,4,5,8-tetraketoneperylenediimide)-4-hexylthiophene was obtained as a yellow solid )-7-fluoro-8-hydroxyquinoline) (2.07 g, 72% yield).
[0049]
[0050] (2) Under nitrogen atmosphere, add compound J (1.72g, 1.5mmol) and I to a 250mL two-necked bottle 2 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com