Asphaltene Components as Organic Electronic Materials
a technology of organic electronic materials and components, applied in the field of organic electronic materials and devices, can solve the problems of reducing the cost-effectiveness associated with the process, and achieve the effects of good intermolecular electronic overlap, good film-forming properties, and high electron affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Example Model of Asphaltene Components
[0033]Calculations were performed on an asphaltene model shown in Scheme 1 (ABC, C125H132N2O3S3). This molecular formula is derived from experimental 1H and 13C NMR, and from mass spectroscopy measurements (Takonahashi T, Sato S, Tanaka R. (2004) Petr. Sci. Tech. 22, 901-914). Previous modeling work has been done under the assumption that the components aggregate around an open, central structure of A (Stoyanov S R, Gusarov S, Kovalenko A. (2008) Mol. Sim. 34, 953-960). This central structure should have a folded form in which the two heterocyclic polyaromatic moieties of A can maximize their stability by π-stacking. Optimization calculations using PBE / 6-31+G(d,p) with DCPs on an open and folded form confirmed this, predicting that the folded form of the aggregate (see FIG. 1) is more stable than the open form by about 9 kcal / mol. NMR work lends support for a closed form for asphaltenes similar to that in FIG. 1.
[0034]It is not straightforward t...
example 2
Experimental Asphaltene Isolation Procedure (Dettman H D, Inman A, Salmon S, Scott K., Fuhr, B. (2005) Energy Fuels 19, 1399-1404.)
[0036]Asphaltenes were precipitated from the D1160 vacuum residues [boiling point (bp)+524° C.] of global crude oils with pentane, using a single treatment of the procedure outlined in Peramanu et. al (Peramanu S, Pruden B P, Rahimi P. (1999) Ind. Eng. Chem. Res. 38, 3121-3130.). This method includes adding 40-volumes of pentane, sonicating in a bath sonicator for 45 min, leaving the mixture to rest overnight at room temperature, then sonicating for an additional 30 min before filtering, and washing with pentane until the eluent is colorless. Trace pentane was removed from the asphaltenes precipitate by heating the asphaltenes to 45° C. in a vacuum oven overnight.
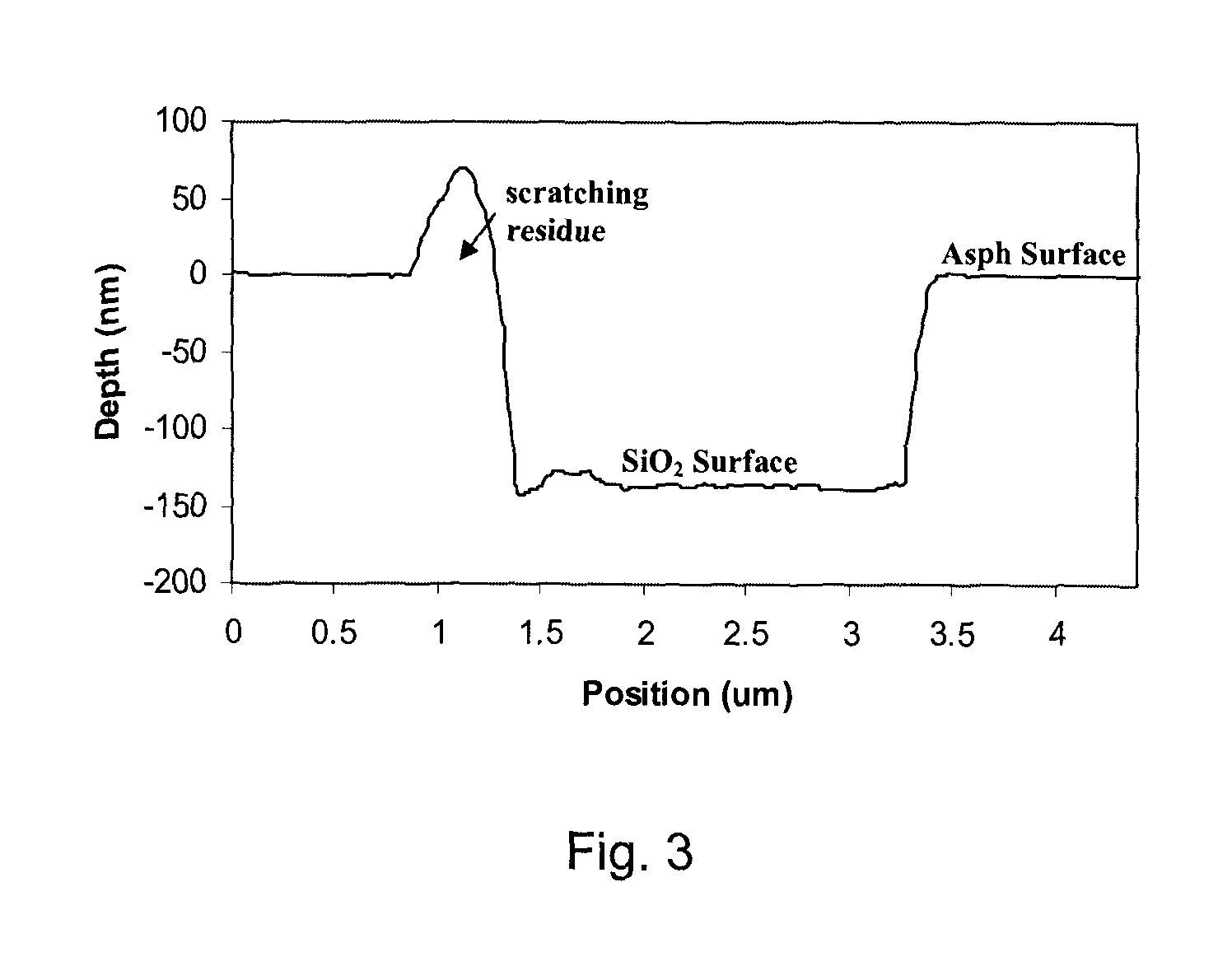
[0037]Gel permeation chromatography was run on the asphaltenes using Bio-beads™ S-X1 purchased from Bio-Rad. These beads are reported to have a molecular weight separation range from 600 to 14,0...
example 3
Asphaltene Experimental Conductance Measurements
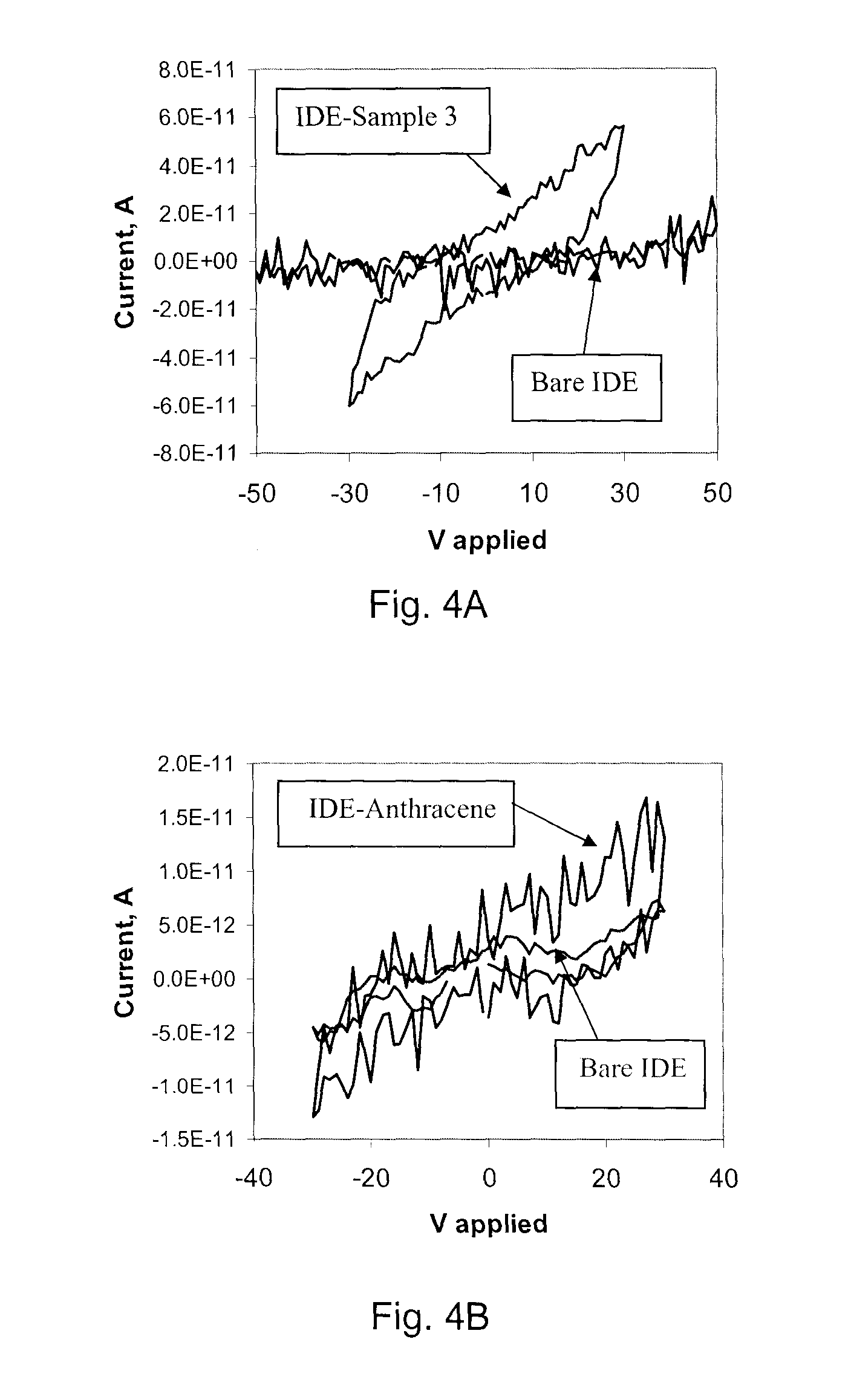
[0039]Three samples of C5 native asphaltene were studied for their electrical conductive properties—Sample 1 consisted of native asphaltene, without component separation; Sample 2 consisted of the early asphaltene fraction (A & B), as acquired from the procedure outlined above; and Sample 3 consisted of the later eluent asphaltenes. The procedure of measuring conductance can be described as thus:
[0040]Asphaltene was dissolved in 2 mL of toluene; the sample spin-coated (1000 rpm for 65 seconds) on a lithography-patterned inter-digitated electrode (IDE, 10 μm separation and 600 digits) on p-Si substrate with 300 nm thermal oxide as an insulating layer. The height of the IDE was 105 nm, constituted by 5 nm Cr (adhesion layer) and 100 nm Au. The sample was dried under vacuum (2×10−6 torr) for 24 hours, with all experimental data collected under vacuum, and in darkness.
[0041]In order that the resistivity of the sample can be determined, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total volume | aaaaa | aaaaa |
| bead size | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com