Nitric oxide in-situ hydrogel for treating colitis
A nitric oxide and hydrogel technology, applied in the directions of anti-inflammatory agent, digestive system, capsule delivery, etc., can solve the problems of carrying, high efficiency, etc., and achieve easy use, good affinity and biocompatibility The effect of convenient sex, storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of nitric oxide in situ hydrogel
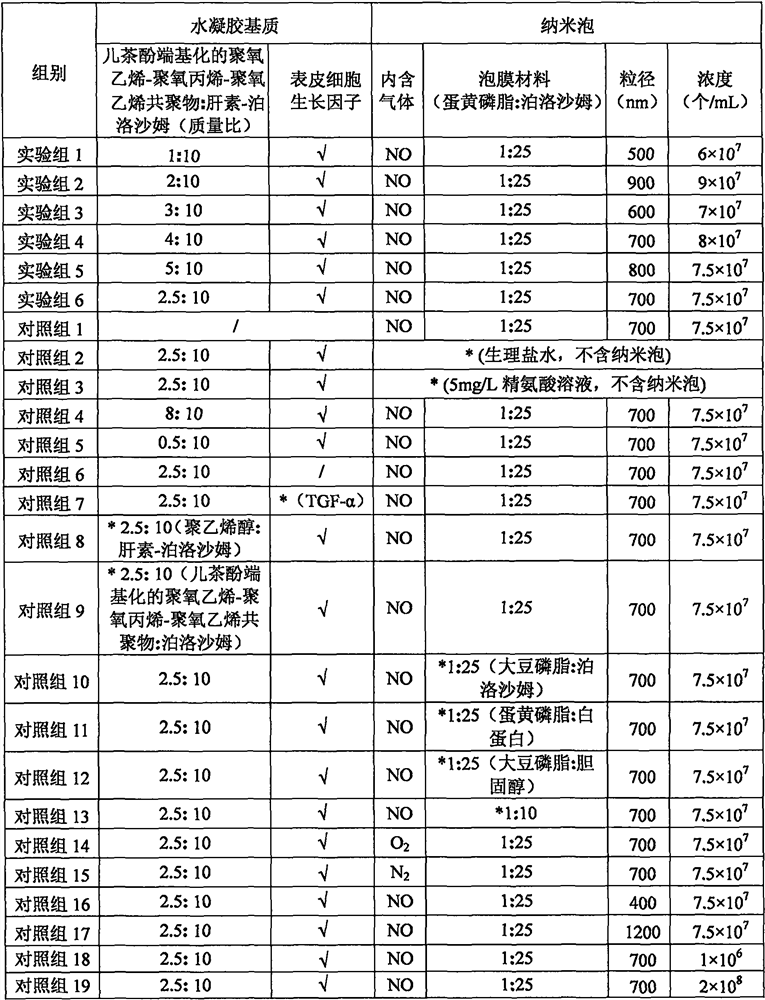
[0025] According to the component ratios in Table 1, the nitric oxide in situ hydrogel of the experimental group was prepared, which specifically included the following steps:
[0026] a: Disperse polyoxyethylene-polyoxypropylene-polyoxyethylene copolymer and heparin-poloxamer in 6 times the mass of 8°C water for injection, place it in a refrigerator at 4-8°C overnight, dissolve slowly, and add 50% epidermal growth factor with equal molar mass of heparin-poloxamer, mixed to form a hydrogel matrix solution;
[0027] b: Take egg yolk phospholipids and poloxamer with a mass ratio of 1:25, mix them, dissolve them in anhydrous tert-butanol 10 times the mass at 65°C, slowly cool down until the solution solidifies, stand overnight at -10°C, and freeze-dry to obtain The loose freeze-dried powder is transferred into a stoppered bottle, filled with nitric oxide gas to saturation, added to water for injection with 5 times t...
Embodiment 2
[0033] Example 2 Application effect of nitric oxide in situ hydrogel for the treatment of colitis
[0034] (1) Establishment of colitis model animals
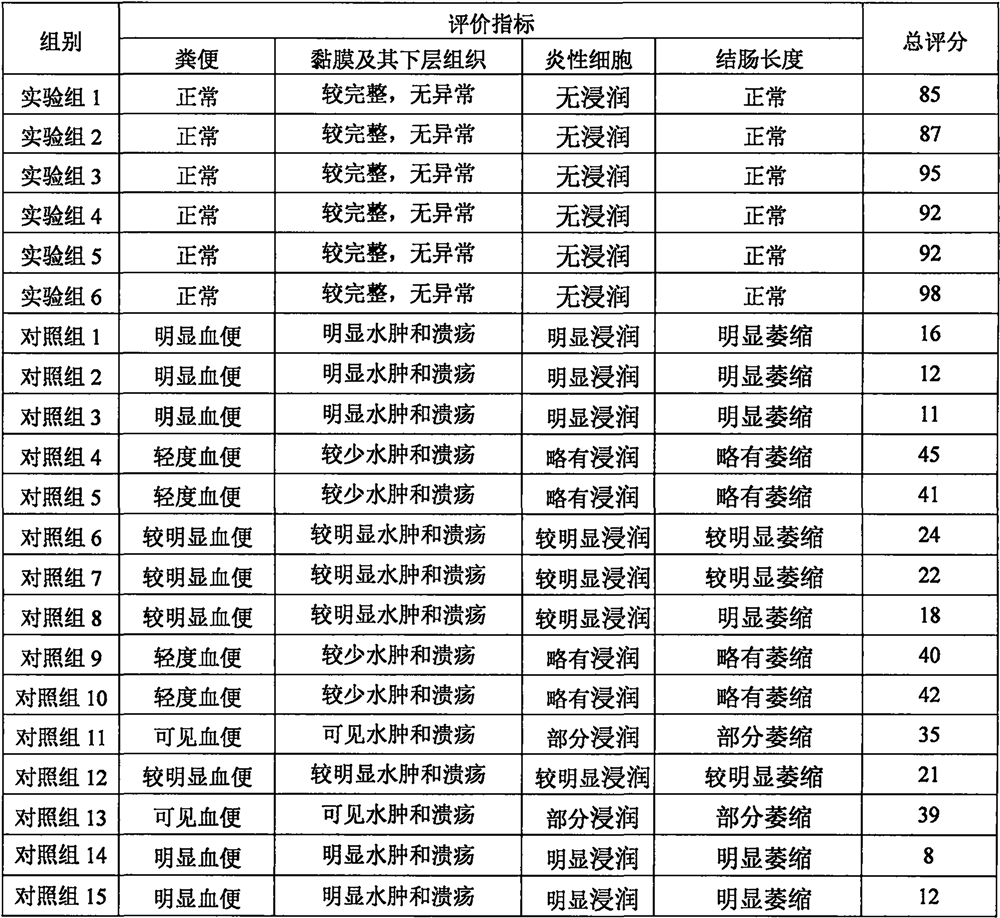
[0035] Male rats (6-8 weeks old) were used as research objects, and colonic perfusion with 2,4,6-trinitrobenzene sodium sulfonate was used to establish colitis model animals, and rat body weight, feces and physical activity were monitored every day . After 3 days, the animal developed diarrhea, mucus, pus and blood in the stool, and it was determined that the modeling was successful.
[0036] (2) Application effect of nitric oxide in situ hydrogel in the treatment of colitis
[0037] Rats with successful colitis modeling were selected and divided into several groups according to the design in Table 1. On days 1, 3, 5, 7, and 9, the colon was perfused with 1 mL of nitric oxide in situ hydrogel, and the rats were fed routinely. Feces were collected on day 12. On the 13th day, the animals were euthanized, and the pathophysiolog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com