Application of nicotinamide mononucleotide in preparation of medicine for preventing, improving and/or treating polycystic ovarian syndrome
A single nucleotide and nicotinamide technology, applied in the field of biomedicine, can solve the problems of no clear standard for the course of treatment, low compliance, and difficulty in effectively improving patients' symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Preparation of PCOS-IR rat model
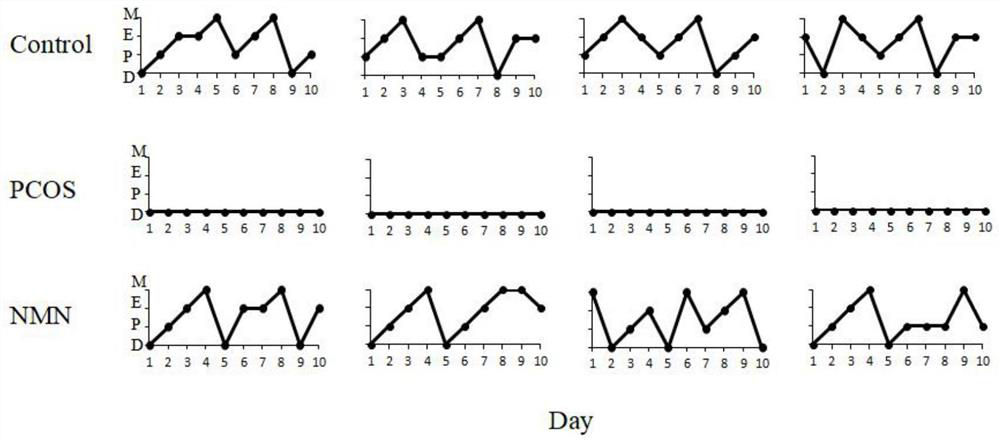
[0056] Five-week-old SD rats were randomly divided into a control group and a model group after one week of adaptive feeding. The model group was treated with trazole 1 mg / kg·d, dissolved in 1% sodium carboxymethyl cellulose (CMC ), continuously gavage for 30 days, while being fed with high-fat feed. The control group was fed with ordinary feed. After 20 days of administration, the pictures of the vagina of SD rats were monitored, and the model was initially determined based on the estrus cycle. The SD rats with successful model construction were divided into PCOS group (model group) and NMN group (experimental group).
Embodiment 2
[0057] Example 2 Preparation of vaginal smear
[0058] The rat was fixed in the supine position, and 20 μL of sterile normal saline was sucked with a pipette, and the normal saline was injected into the vagina about 1 cm. The pipette was repeatedly blown and sucked 3 times, and 10 μL of liquid was sucked out and smeared on the slide evenly, and dried. Add 1-2 drops of Wright-Giemsa stain solution to each slide. After 2 minutes, add the same dose of 0.01M disodium hydrogen phosphate buffer. Shake the slide to make the two liquids evenly mixed. Let stand for 10 minutes. Rinse the dye solution with distilled water. After drying, the xylene dye solution is transparent and sealed with neutral resin for microscopic inspection.
Embodiment 3
[0059] Example 3 Blood sampling from the abdominal aorta and ovarian and pancreatic tissue sampling
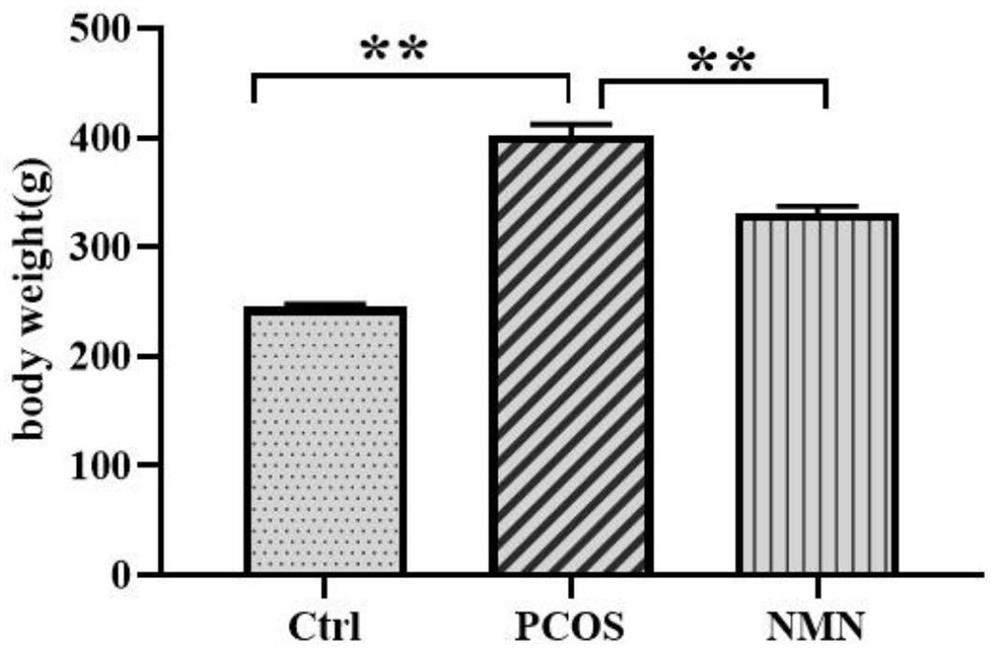
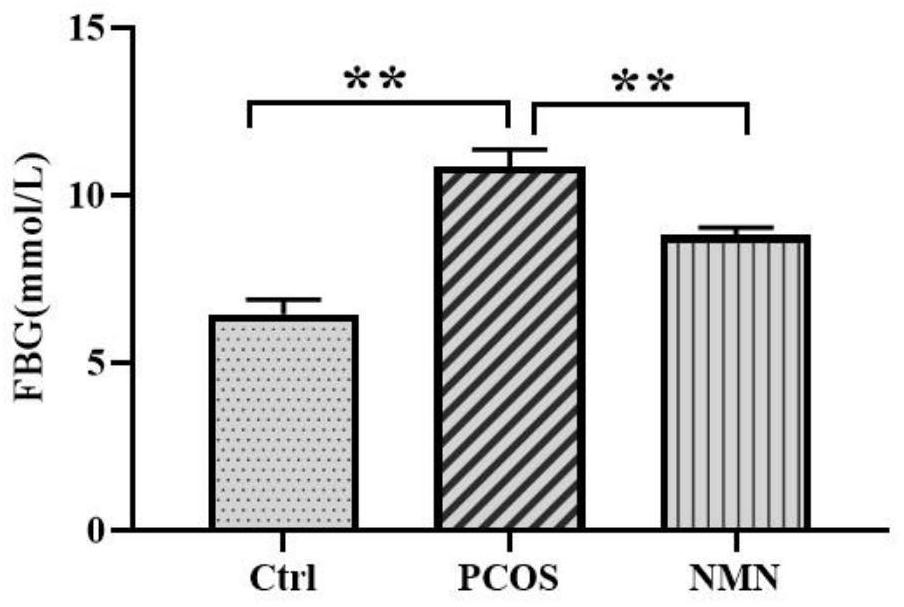
[0060] The rats were treated after the expiration of the administration period and fasted for 12 hours before treatment. All rats were weighed, and blood was collected from the tail vein to measure fasting blood glucose. Chloral hydrate was used for intraperitoneal anesthesia (6% concentration, 0.6-0.7ml / 100g body weight). After successful anesthesia, fix the rat on the dissection table in a supine position. Shave the abdomen. After disinfection, cut it layer by layer along the main midline of the rat's abdomen with scissors. After opening the abdominal cavity, look for the abdominal aorta and abdominal aorta Located in front of the spine and beside the abdominal vena cava (thicker and darker in color than the abdominal aorta), use forceps or hemostatic forceps to gently separate the adipose tissue and fascia tissue around the blood vessels to clearly expose the blood vessels. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com