Rapamycin nanoparticle for use in porous percutaneous transluminal angioplasty
An angioplasty, rapamycin technology, applied in cardiovascular system diseases, catheters, capsule delivery and other directions, can solve problems such as increased inflammatory response, low drug utilization, loss of drugs, etc., to prevent vascular restenosis, preparation method Simple, Affinity Enhancement Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
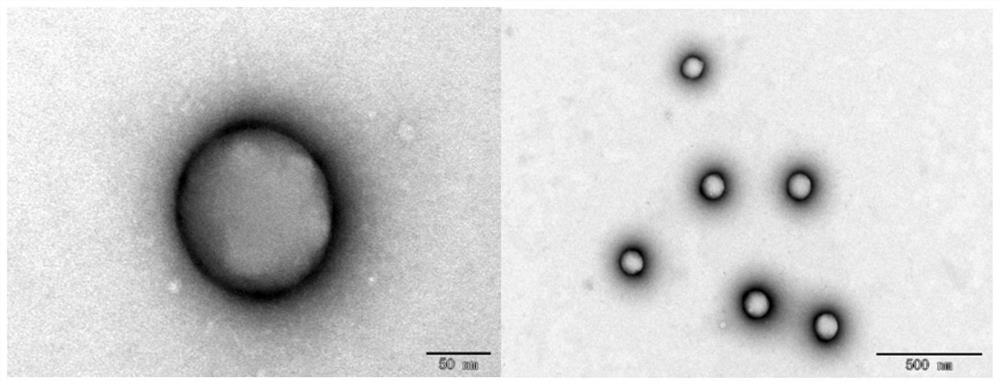
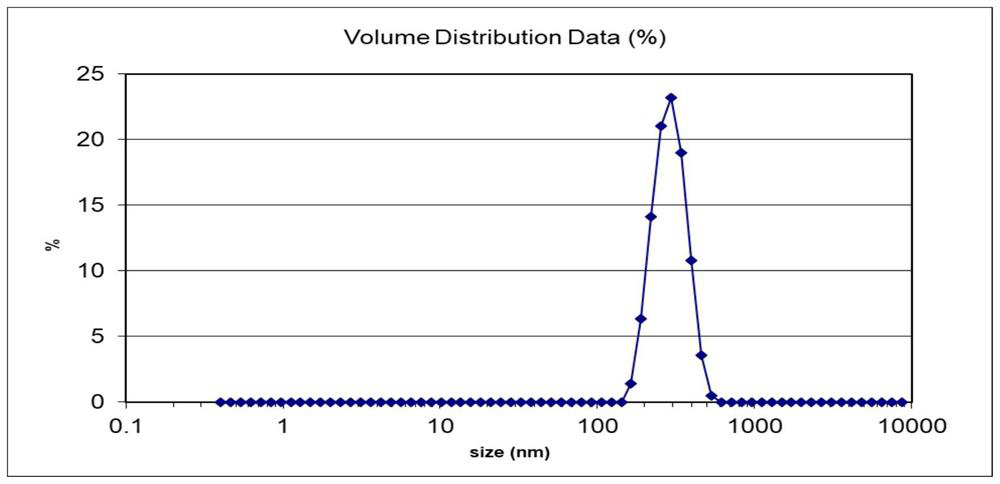
[0028] Using acetonitrile as the organic phase to prepare rapamycin lipopolymer hybrid nanoparticles, the particle size is about 365.6nm, spherical nanoparticles with a potential of about -20.2mV, see figure 1 and figure 2 . The encapsulation rate is 91.5%, and the specific implementation method is as follows:
[0029] (1) Dissolve 0.02 g of rapamycin and 0.16 g of polylactic acid in 32 mL of acetonitrile as an oil phase. Dissolve 0.01 g of DPPC and 0.006 g of PEGylated phospholipids in 320 mL of 4% aqueous ethanol and heat to 65° C. to ensure that all lipids are dissolved as the aqueous phase.
[0030] (2) Add the oil phase dropwise into the preheated lipid solution at a certain speed to form an O / W single emulsion. Using an ultrasonic cell disruptor, sonicate in an ice bath to form a homogeneous emulsion with smaller particle size.
[0031] (3) The organic solvent is evaporated to dryness, and the organic solvent can be recycled. The precipitate was centrifuged and was...
Embodiment 2
[0033] Using dichloromethane as the organic phase to prepare rapamycin lipopolymer hybrid nanoparticles, the obtained particle size is about 280.1nm, PDI is 0.089, and the potential is a spherical nanoparticle of -13.5mV, see image 3 and Figure 4 . The encapsulation rate is 89.6%, and the specific implementation method is as follows:
[0034] (1) Dissolve 0.02 g of rapamycin, 0.16 g of polylactic acid, 0.01 g of DPPC, 0.003 g of PEGylated phospholipid, and 0.003 g of cationic phospholipid in 32 mL of dichloromethane as an oil phase. Prepare 1% PVA aqueous solution, take 320mL as the water phase for later use.
[0035] (2) Add the oil phase dropwise into the stirring water phase at a certain speed to form an O / W single milk emulsion. Under ice bath, use a high-pressure homogenizer to form a uniform emulsion with smaller particle size.
[0036] (3) The organic solvent is evaporated to dryness, and the organic solvent can be recycled. The precipitate was centrifuged and wa...
Embodiment 3
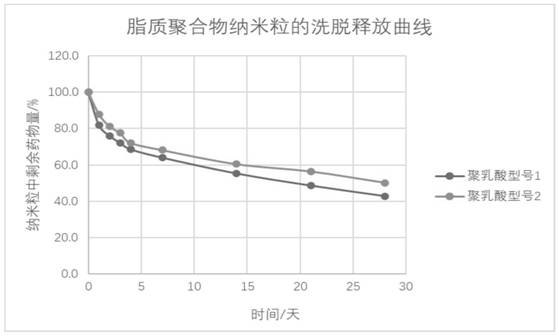
[0038] Using the method in Example 2, two types of polylactic acid were used as polymers to prepare lipopolymer nanoparticles, and the in vitro release rule was investigated.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com