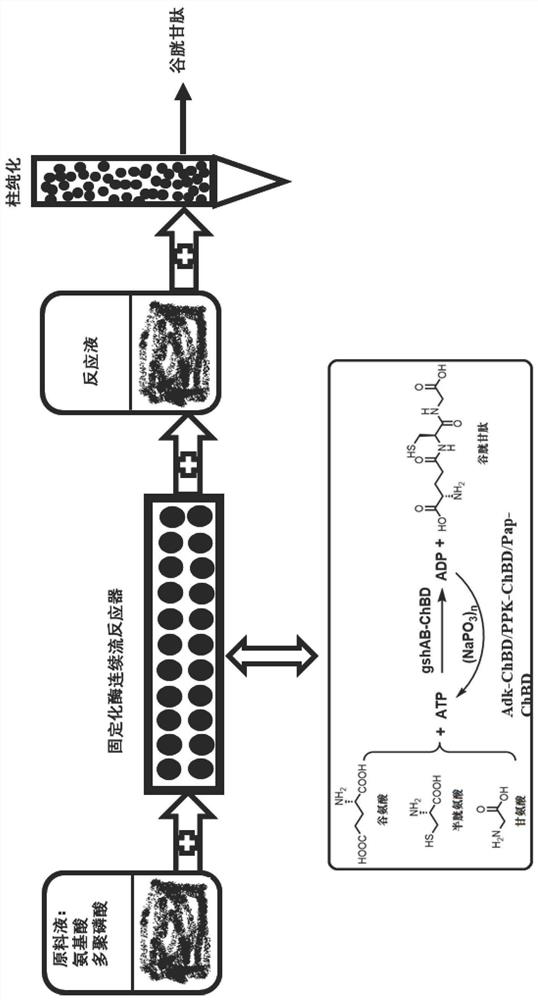
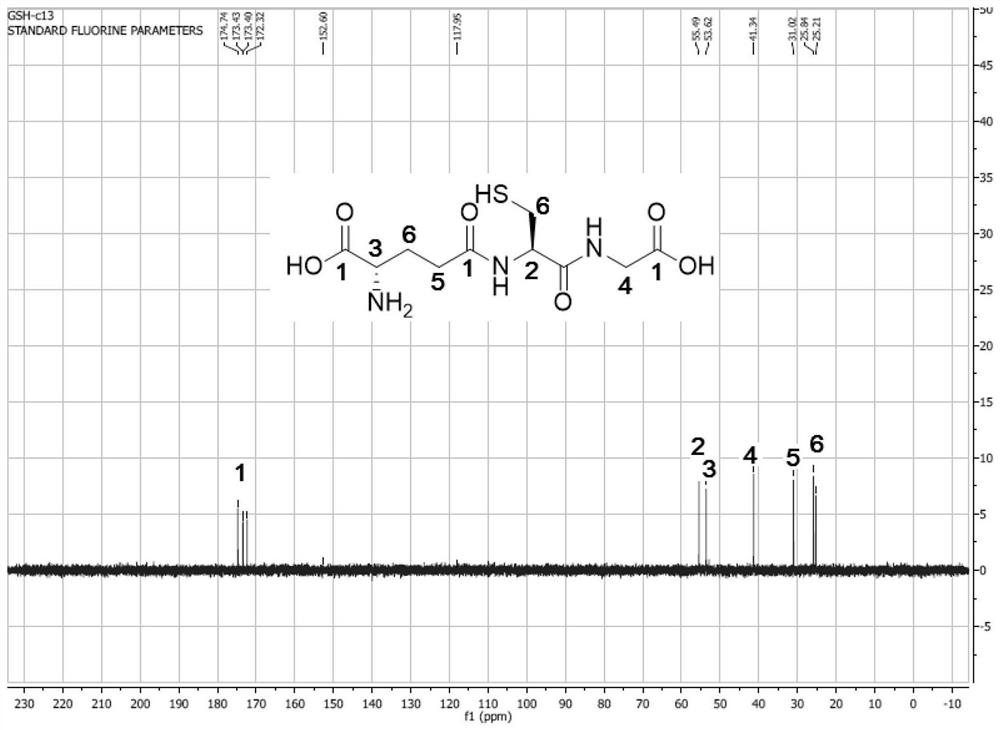
Immobilized Fusion Enzyme and Method for Using It to Prepare Glutathione
A glutathione and fusion enzyme technology, applied in biochemical equipment and methods, fusion polypeptides, immobilized on/in organic carriers, etc. Final product purification and other issues, to achieve the effect of high immobilization unit activity density, high target product concentration, and improved substrate conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
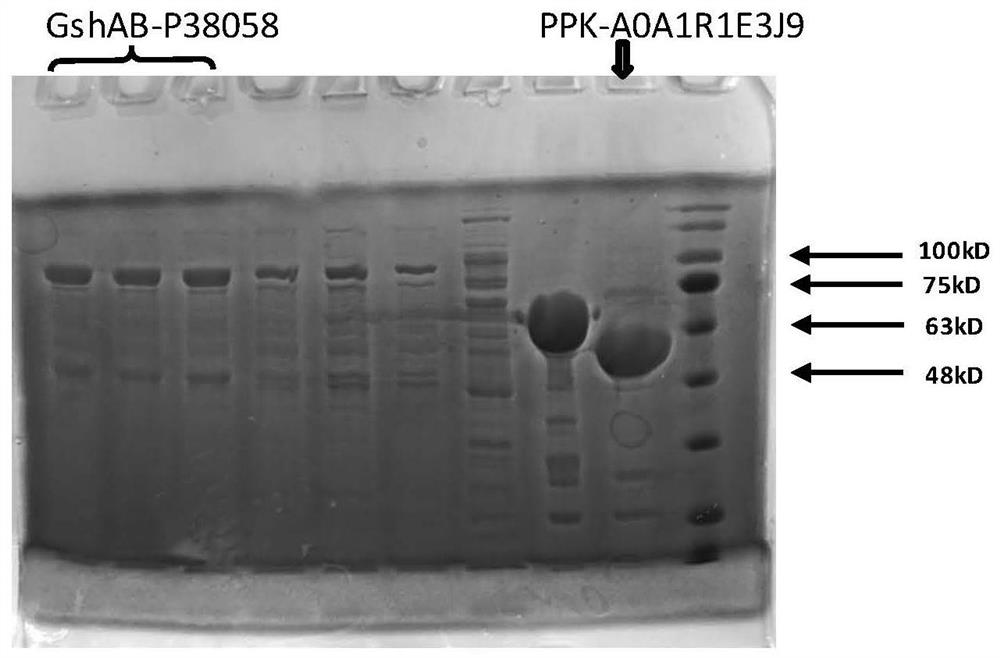
[0061] The fusion protein expression gene (GshAB-P38058) of glutathione bifunctional enzyme (GshAB) EC 6.3.2.2 and chitin protein binding domain (ChitinBinding Domain, P38058 derived from Clostridium cellulovorans) was constructed. The protein sequence of GshAB-P38058 is shown in Table 1. After the gene was synthesized, it was subcloned into pColdIII (TaKaRa, Japan) plasmid by NdeI and XhoI (NEB Company) digestion.
[0062] Construct polyphosphate kinase (PPK, EC 2.7.4.1) and chitin protein binding domain (Chitin BindingDomain, A0A1R1E3J9 derived from Paenibacillus amylolyticus), PPK-A0A1R1E3J9 protein sequence is shown in Table 1, after gene synthesis by NdeI and XhoI (NEB company ) and subcloned into pColdIII (TaKaRa, Japan) plasmid.
[0063] Adenylate kinase (Adk, EC 2.7.4.3) and chitin protein binding domain (Chitin BindingDomain, A0A089MPB0 derived from Paenibacillus odorifer) were constructed. The protein sequence of Adk-A0A089MPB0 is shown in Table 1. ) and subcloned i...
Embodiment 2
[0075] The fusion protein expression gene (GshAB-4B9F) of glutathione bifunctional enzyme (GshAB) EC 6.3.2.2 and chitin protein binding domain (ChitinBinding Domain, Clostridium Thermocellum-derived 4B9F) was constructed. See attached table 1 for the protein sequence of GshAB-4B9F. After gene synthesis, it was subcloned into pColdIII (TaKaRa, Japan) plasmid by NdeI and XhoI (NEB Company) digestion.
[0076] Construct polyphosphate kinase (PPK, EC 2.7.4.1) and chitin protein binding domain (Chitin BindingDomain, A0A1R1E3J9 derived from Paenibacillus amylolyticus), PPK-A0A1R1E3J9 protein sequence is shown in Table 1, after gene synthesis by NdeI and XhoI (NEB company ) and subcloned into pColdIII (TaKaRa, Japan) plasmid.
[0077] GshAB-4B9F wet cells and PPK-A0A1R1E3J9 wet cells were prepared by inducing expression of the above plasmids according to the method of Example 1.
[0078] Weigh 100g GshAB-4B9F wet cells, resuspend in 1000ml solution (containing 20mM Tris pH7.5) and c...
Embodiment 3
[0082] Construct the fusion protein expression gene (GshA-A0A173MZQ9) of glutathione monofunctional synthetase GshA (EC 6.3.2.2) and chitin protein binding domain (source A0A173MZQ9). The protein sequence of GshA-A0A173MZQ9 is shown in Table 1. After the gene was synthesized, it was subcloned into pColdIII (TaKaRa, Japan) plasmid by NdeI and XhoI (NEB Company) digestion.
[0083] The fusion protein expression gene (GshB-A0A173MZQ9) of glutathione monofunctional synthetase GshB (EC 6.3.2.3) and chitin protein binding domain (source A0A173MZQ9) was constructed. See attached table 1 for the protein sequence of GshB-A0A173MZQ9. After the gene was synthesized, it was subcloned into pColdIII (TaKaRa, Japan) plasmid by NdeI and XhoI (NEB Company) digestion.
[0084]Construct polyphosphate kinase (PPK, EC 2.7.4.1) and chitin protein binding domain (Chitin BindingDomain, 4B9F derived from Clostridium Thermocellum). The sequence of PPK-4B9F protein is shown in Table 1. After gene synthe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com