Synthesis method of alpha-aluminum trihydride
A synthesis method, aluminum hydride technology, applied in chemical instruments and methods, metal hydrides, inorganic chemistry, etc., can solve problems such as difficult purification, moisture sensitivity, etc., achieve easy control of reaction, convenient operation, and reduce equipment requirements Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The present invention provides a kind of synthetic method of α-aluminum trihydride, comprises the following steps:
[0018] Step 1: Add bis(bromomethyl)benzene to an anhydrous ether solution of lithium aluminum hydride, and react under an inert atmosphere, and obtain an ether complex of aluminum trihydride after the reaction is completed.
[0019] In a preferred embodiment, the bis(bromomethyl)benzene is selected from 1,2-bis(bromomethyl)benzene, 1,3-bis(bromomethyl)benzene or 1,4-bis( Any one or more of bromomethyl)benzene.
[0020] In a preferred embodiment, LiAlH 4 The ratio of the amount of the substance to the volume of anhydrous ether is 1mol: 1.4L ~ 4.8L.
[0021] In a preferred embodiment, LiAlH 4 The molar ratio (that is, the ratio of the amount of substances) to bis(bromomethyl)benzene is 2.1-3.0:1.
[0022] In a preferred embodiment, the reaction is carried out at 5-30°C for a reaction time of 2-24 hours, with stirring during the reaction; preferably, the...
Embodiment 1
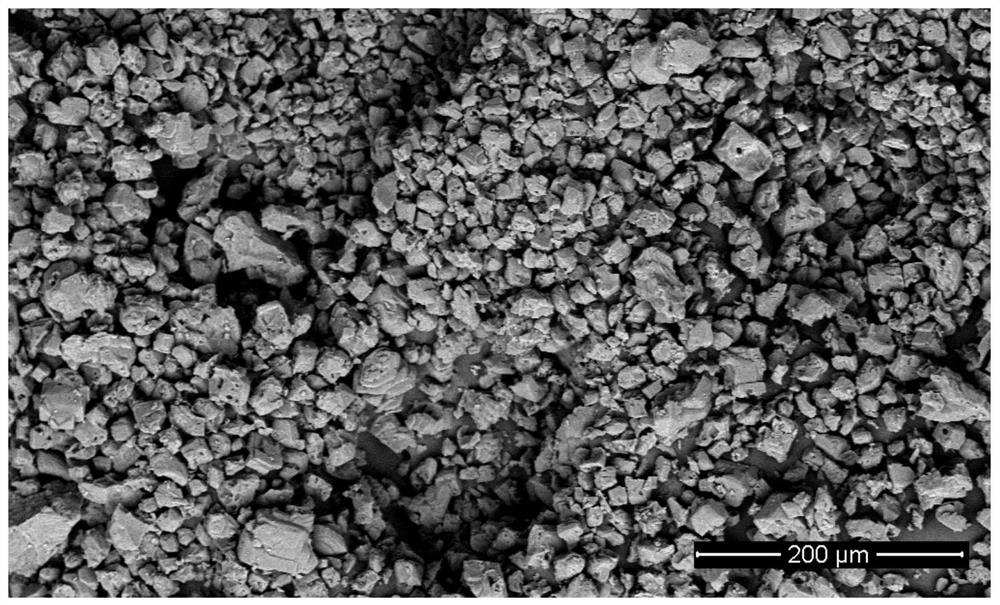
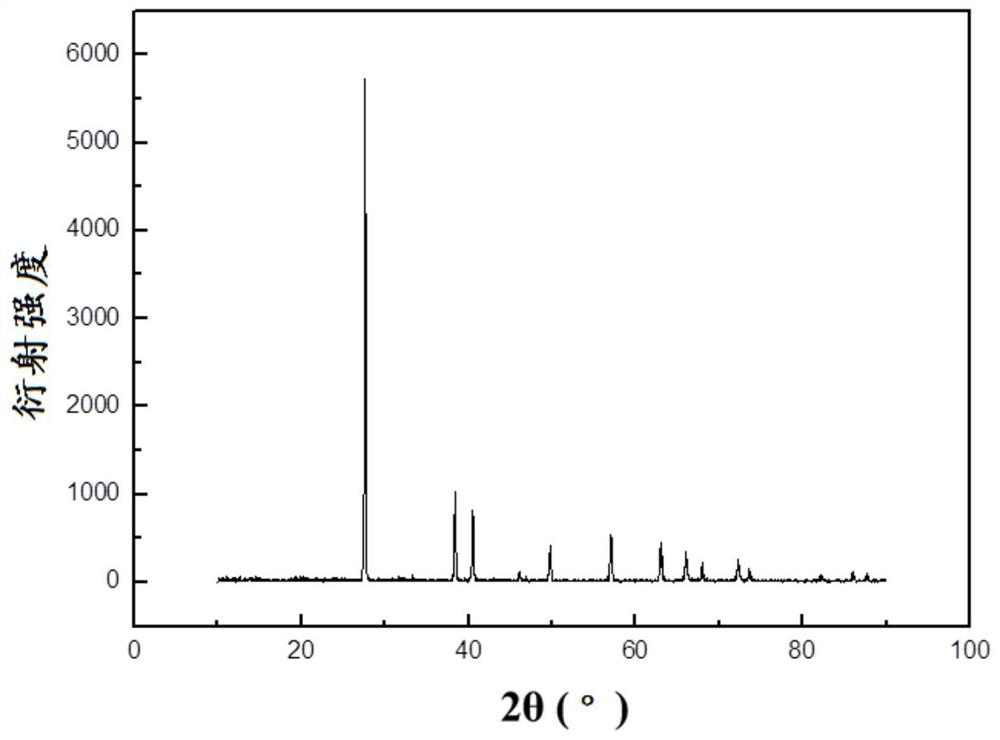
[0030] 9.5 g LiAlH 4 (0.25mol) was dissolved in 1L of anhydrous ether, protected by nitrogen, added 26.4 grams of 1,2-bis(bromomethyl)benzene (0.1mol), stirred and reacted at 15°C for 2 hours, and transferred the reaction solution to 82°C Carry out ether removal and crystallization in 0.7L of toluene. After no diethyl ether is distilled out in the condenser, the reaction system is lowered to room temperature, filtered, and then washed with ethanol. After drying at normal pressure at 50°C, 4.32 grams of α-AlH are obtained. 3 product. α-AlH 3 The SEM electron microscope picture is as figure 1 As shown, the X-ray diffraction pattern is as figure 2 shown.
Embodiment 2
[0032] 15.2 g LiAlH 4 (0.4mol) was dissolved in 1L of anhydrous ether, protected by nitrogen, added 39.6 grams of 1,3-bis(bromomethyl)benzene (0.15mol), stirred and reacted at 20°C for 4 hours, and transferred the reaction solution to 83°C Carry out ether removal and crystallization in 2L of toluene. After no diethyl ether is distilled out in the condenser, the reaction system is cooled to room temperature, filtered, and then washed with diethyl ether. After drying at normal pressure at 40°C, 7.29 grams of α-AlH are obtained. 3 product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com