Synthesis and impurity identification method of bromhexine hydrochloride process impurity positioning reference substance
A technology of bromhexine hydrochloride and process impurities, which is applied in the field of quality inspection of bromhexine hydrochloride, can solve the problem of lack of impurity positioning reference substances and impurity structure confirmation of process impurity by-products, difficulty in monitoring drug quality, and limited quality control of bromhexine hydrochloride. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
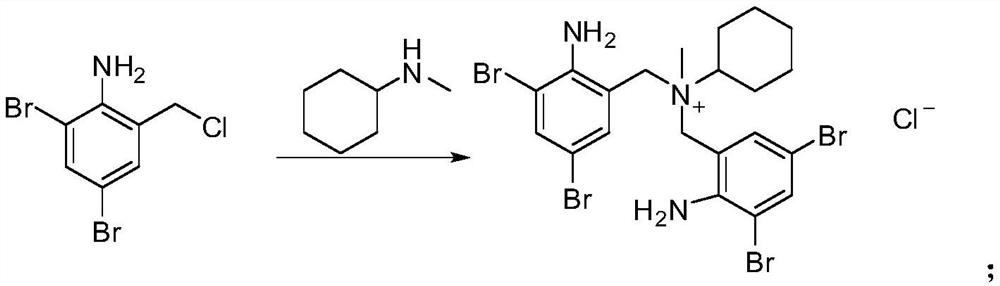
[0017] A kind of synthetic method of bromhexine hydrochloride process impurity, its synthetic route is as follows:
[0018]
[0019] The specific synthesis steps are: add 50g of N-methylcyclohexylamine to the reaction flask, cool down to 10°C, add 100g of 2,4-dibromo-6-chloromethylaniline in batches and control the addition temperature to be less than or equal to 50°C until the Complete, keep the temperature at 45-50°C for stirring reaction for 3h, add 300ml of ethanol to continue stirring and keep the temperature at 45-50°C for 1h, filter while hot and collect the filtrate into the reaction bottle, add 200ml of ethanol and drop hydrochloric acid After adjusting the pH to 4, stir at room temperature for 12 hours, take the concentrated filtrate by suction filtration, separate the slightly viscous product by column chromatography, add acetonitrile to solidify, and dry to obtain N,N-bis(2-amino- 3,5-dibromophenyl)-N-methylcyclohexyl ammonium chloride 14g, as the impurity posit...
Embodiment 2
[0021] An identification method of bromhexine hydrochloride process impurities, its identification method is as follows: Synthesize N, N-di(2-amino-3,5-dibromophenyl)-N-methanol according to any one of the above synthetic methods Cyclohexyl ammonium chloride is used as a reference substance for positioning; when preparing bromhexine hydrochloride, the material obtained by column chromatography separation from the mother liquor is slightly viscous, solidified, filtered, and dried to obtain a light yellow solid process impurity sample. Resonance spectrum and liquid phase-mass spectrometry for qualitative analysis of positioning reference substances and process impurity samples:
[0022] When using Bruker AvanceⅢ400 nuclear magnetic resonance instrument for nuclear magnetic resonance spectrum analysis: deuterated methanol MeOD is used as the test solvent to prepare process impurity samples and place them in nuclear magnetic tubes; measurement temperature: room temperature 20-35 °C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com