Subunit vaccine for preventing novel coronavirus infection based on bur protein S1 region of novel coronavirus
A subunit vaccine, coronavirus technology, applied in the field of vaccines, to achieve the effect of avoiding ADE risk and low ADE risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
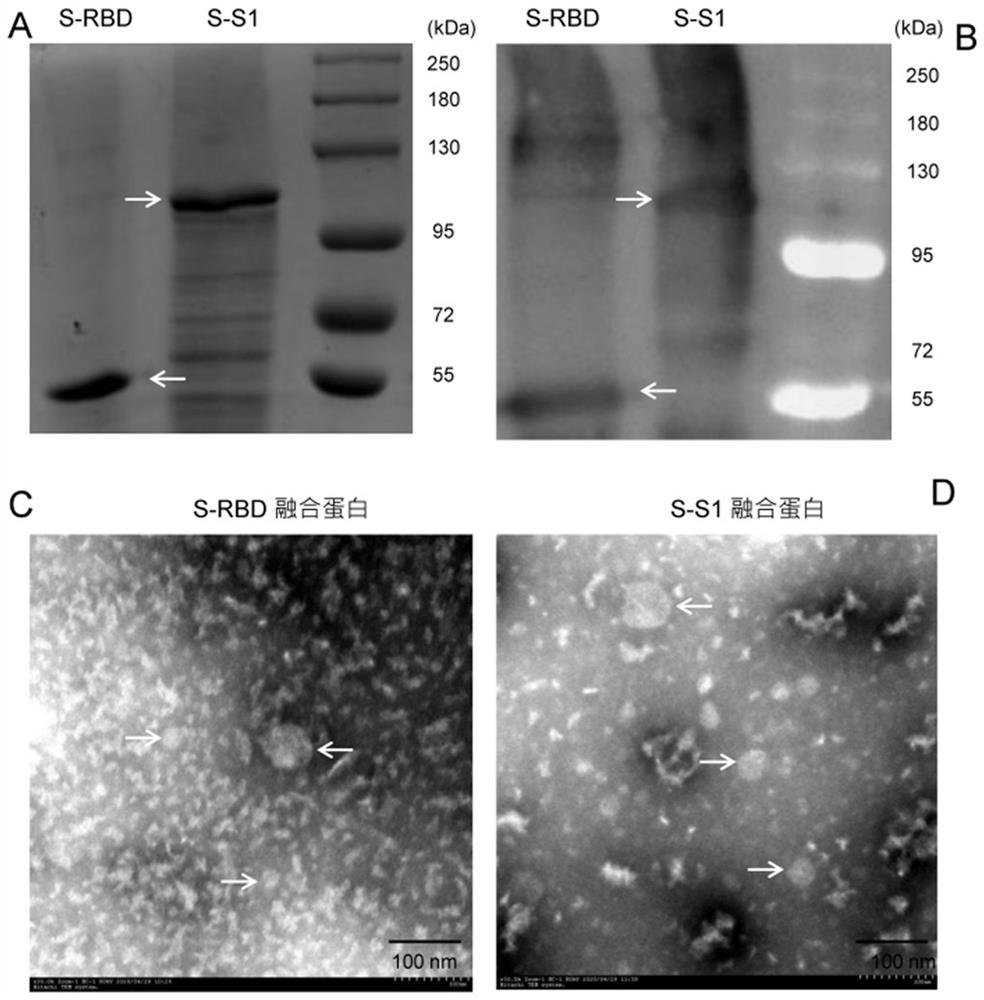
[0024] Example 1 Preparation of antigen
[0025] The gene encoding a new coronary virus S1 region (YP_009724390.1, MET1-TYR695) and RBD regions (YP_009724390.1, ARG328-PRO521) gene is connected to genes encoding the Northern, in accordance with the E. coli codon preferences, by Nanjing Jinsuri synthesis, constructed between BamHI and NOTI-dug sites of plasmid PET-28B, transformed E. coli (BL-21, DE3), 0.4 mM isopropyl-β-D-thiosani Lactoside at room temperature (~ 25 ° C) induces overnight. 4 ° C 8000 rpm was centrifuged for 15 min to collect the bacteria, resuspended in the PBS solution Ultrasound 15 min, 4 ° C 8000 rpm centrifugation 60 min to collect precipitation, ultrasonically cleaning 15 min, 4 ° C 8000 rpm centrifugal 15 min collection inclusion body dissolved in 8 m urea Dialysis of 20 mm Tris-HCl (pH 7.4) solution containing 6 mm, 4 m, 1 m and 0 m was used in 4 ° C. The prepared protein uses a TCA protein concentration test kit (Biyunndian) to determine concentration, 10%...
Embodiment 2
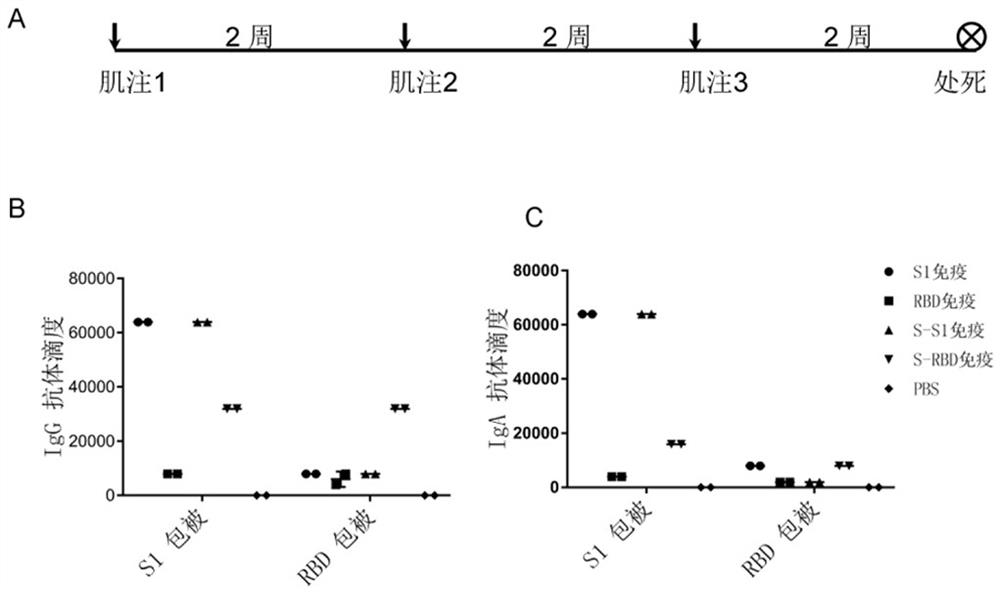
[0027] Example 2 - Animal Immunity
[0028]S1 and RBD protein (Qiqiao) expressed by mammalian cell HEK293, and E. coli expression, E. coli, is diluted with 10 μg / 25 μL, mixed with the same volume of alumina. After the agent (40 mg / ml), the interval was 2 peripheral injection of immunized BALB / C mice (6 weeks old, 14-17 grams, purchased by the Institute of Medicine, Chinese Academy of Sciences, the experimental animal center). After 2 weeks, the heart was centrifuged at 4 ° C after 2 weeks after 3 000 rpm was centrifuged for 10 minutes to take blood, preparing for subsequent immunological analysis.
[0029] Among them, the amino acid sequence of the S1 protein is:
[0030] VNLTTRTQLPPAYTNSFTRGVYYPDKVFRSSVLHSTQDLFLPFFSNVTWFHAIHVSGTNGTKRFDNPVLPFNDGVYFASTEKSNIIRGWIFGTTLDSKTQSLLIVNNATNVVIKVCEFQFCNDPFLGVYYHKNNKSWMESEFRVYSSANNCTFEYVSQPFLMDLEGKQGNFKNLREFVFKNIDGYFKIYSKHTPINLVRDLPQGFSALEPLVDLPIGINITRFQTLLALHRSYLTPGDSSSGWTAGAAAYYVGYLQPRTFLLKYNENGTITDAVDCALDPLSETKCTLKSFTVEKGIYQTSNFRVQPT...
Embodiment 3
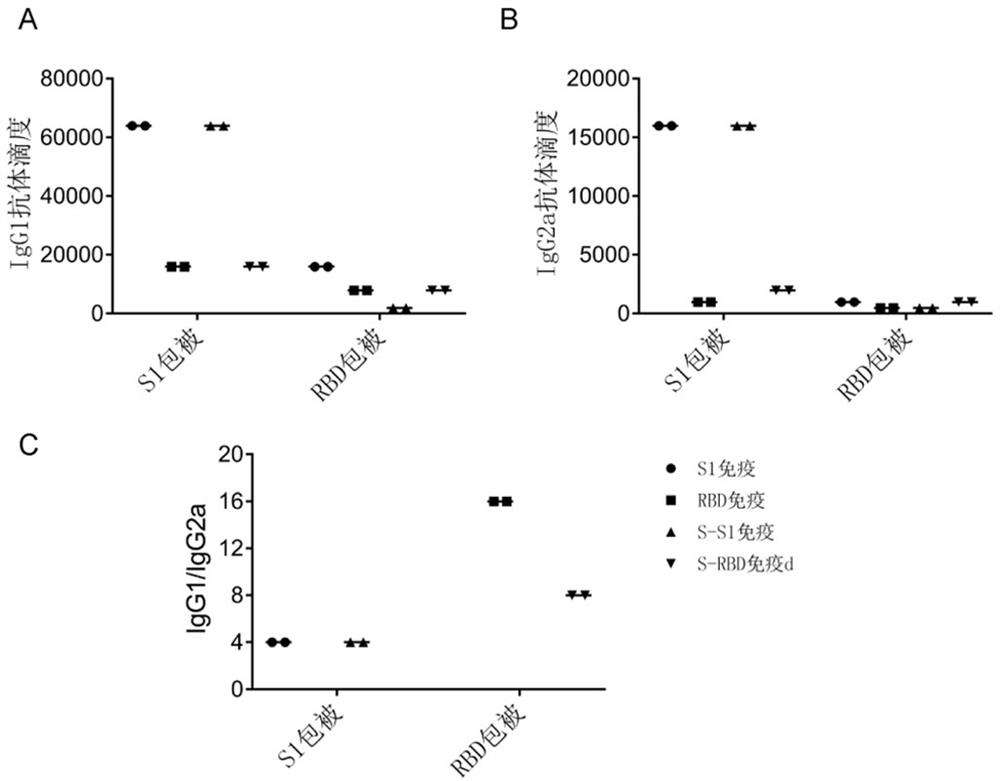
[0032] Example 3 - Antibody titer detection
[0033] S1 and RBD protein 2 μg / ml expressed in PBS were added to S1 and RBD protein 2 μg / ml per well to add 96-pole enzyme board (Corning), 4 ° C overnight package was subjected to TBST (0.05% Tween 20 (Sigma) in PBS 1 time, 200 μl of 5% (w / v) is added to PBS per hole 37 ° C to close 1 h, abandoned TBST was washed 4 times, 100 μL of 100 μL per well was added to 1% milk gradient dilution Example 2 Anti-Ease 37 ° C was incubated for 1 h, TBST was washed 5 times, and 1% milk diluted horseradish peroxidase labeled second anti-sheep anti-mouse IgG / IgA / IgG1 / IgG2A (Saimi fly) 37 ° C After incubation of 1 h, TBST washed 5 times, 100 μL per well was added to 1: 1-based color culture (purchased from BD), and then protected from light at room temperature for 10 minutes, and 100 μl of 2 m sulfuric acid to terminate each well. The light absorption value is detected at 450 nm. Serum dilution concentrations critical in OD450 <0.1 were tak...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com