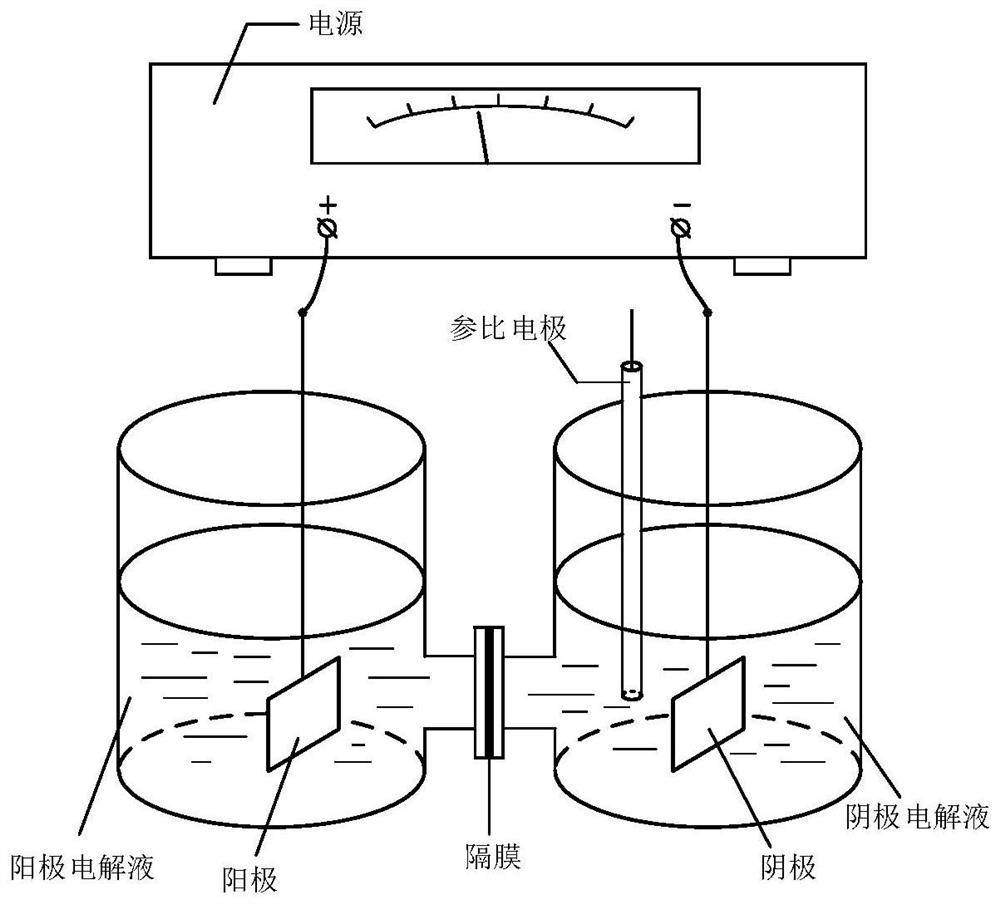
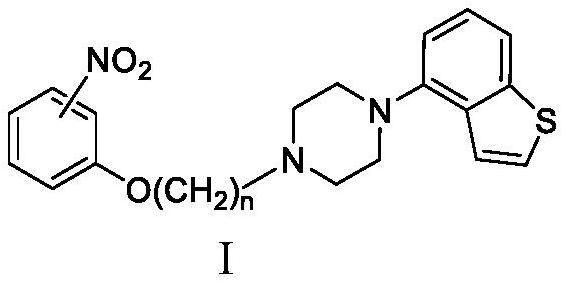
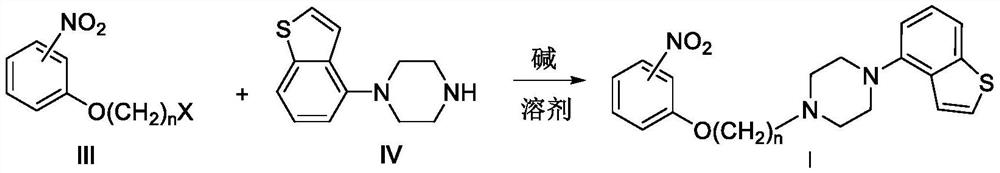
Piprazole antipsychotic drug key intermediate and electroreduction method thereof
An intermediate and a key technology, applied in the application field of preparing Kang psychiatric drugs, can solve the problems of increasing the two-step synthesis reaction, reducing the total yield, and being unfriendly to the environment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of 1-(4-bromobutoxy)-3-nitrobenzene
[0049]
[0050] Add 20mmol 3-nitrophenol and 24mmmol potassium carbonate to 30ml N,N-dimethylformamide, stir at room temperature for 1h, then add 32mmol 1,4-dibromobutane to the mixture and react for 16.0h; the reaction solution is poured into 200ml of water, 100ml of dichloromethane extracted 3 times, combined organic phase, washed 3 times with 100ml of 2% sodium hydroxide solution, washed 2 times with 100ml of water; dried with anhydrous sodium sulfate; recovered solvent by rotary evaporation, and used petroleum ether-ethyl acetate Esters (V 石油醚 :V 乙酸乙酯 =50:1) column chromatography, purified to obtain 3.70g 1-(4-bromobutoxy)-3-nitrobenzene, yield 67.5%; 1 H NMR (400MHz, CDCl 3 )δ: 7.88—7.84 (m, 1H, C 6 h 4 ), 7.76(t, J=2.3Hz, 1H, C 6 h 4 ), 7.47(t, J=8.2Hz, 1H, C 6 h 4 ), 7.25 (dd, J = 8.6Hz, 2.1Hz, 1H, C 6 h 4 ), 4.12(t, J=6.4Hz, 2H, OCH 2 ), 3.54(t, J=6.4Hz, 2H, CH 2 ), 2.15—2.02 (m, 4H, CH 2 CH 2 )...
Embodiment 2
[0052] Preparation of 1-(4-chlorobutoxy)-3-nitrobenzene
[0053]
[0054] Add 20mmol 3-nitrophenol and 24mmmol potassium carbonate to 30ml N,N-dimethylformamide, stir at room temperature for 1h, then add 20mmol 1-bromo-4-chlorobutane to the mixture for overnight reaction for 16h; Pour the solution into 200ml water, extract three times with 100ml dichloromethane, combine the organic phases, wash three times with 100ml of 2% sodium hydroxide solution, and wash twice with 100ml water; dry the organic phase with anhydrous sodium sulfate; Solvent, using petroleum ether: ethyl acetate = 50: 1 column chromatography, purified to obtain 3.26g 1-(4-chlorobutoxy)-3-nitrobenzene, yield 71.0%; 1 H NMR (400MHz, CDCl 3 )δ: 7.88—7.84 (m, 1H, C 6 h 4 ), 7.76(t, J=2.3Hz, 1H, C 6 h 4 ), 7.47(t, J=8.2Hz, 1H, C 6 h 4 ), 7.25 (dd, J = 8.6Hz, 2.1Hz, 1H, C 6 h 4 ), 4.12(t, J=6.4Hz, 2H, OCH 2 ), 3.54(t, J=6.4Hz, 2H, CH 2 ), 2.15—2.02 (m, 4H, CH 2 CH 2 ).
Embodiment 3
[0056] Preparation of 1-(Benzo[b]thiophen-4-yl)-4-(4-(3-nitrophenoxy)butyl)piperazine
[0057]
[0058] 10mmol 1-(4-bromobutoxy)-3-nitrobenzene, 10mmmol1-(2,3-dichlorophenyl)piperazine hydrochloride and 12mmol potassium carbonate were added to 25ml acetonitrile, and refluxed for 48h. Inorganic matter is removed, the filtrate is washed, the filtrate is concentrated, and the organic solvent is recovered. Add 30ml of dichloromethane and 10ml of water to the concentrate, extract the aqueous layer 3 times with 20ml of dichloromethane, combine the organic phases, and wash with water until neutral. Dry the organic phase with anhydrous sodium sulfate, filter with suction; use V 石油醚 :V 乙酸乙酯 =10:1 column chromatography, to obtain 2.41g 1-(benzothienyl)-4-(4-(3-nitrophenoxy)butyl)piperazine; yield 58.6%; 1 H NMR (400MHz, CDCl 3 )δ: 7.86 (d, J=9.2Hz, 1H, C 6 h 4 ), 7.78(t, J=2.1Hz, 1H, C 6 h 4 ), 7.60 (d, J=8.0Hz, 1H, C 6 h 4 ), 7.49~7.45(m, 2H, SCH=CH), 7.43(d, J=5.6Hz, 1H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com